Meeting Summary
The third Encuentro Latinoamericano de Infecciones Respiratorias Recurrentes (ELAIR) took place in Mexico City, Mexico, on 11th−12th May 2017. ELAIR brought together experts from across Latin America and further afield, continuing an extraordinary didactic exercise on the cutting-edge advances of respiratory medicine. Impressive progress has been made in the past 15 years, with new treatments available to manage and prevent airway infections. It remains to be seen how this might affect the related conditions of wheezing and asthma in predisposed and sensitised subjects. However, early data suggest that lower respiratory infection rates may reduce the development of the above conditions which are closely related to viral infections. Immunomodulators that both prime the immune system to fight infection and reduce inflammation are likely to play a major role in secondary and even potentially primary prevention of atopic diseases.
ASTHMA PATHOPHYSIOLOGY: ROLE OF RESPIRATORY TRACT INFECTIONS
Asthma is a major public health problem with a conservative global prevalence of 235 million people. The economic (>$18 billion annually in the USA) and humanitarian burden of asthma falls more heavily on developing countries, where management tends to be poorer, increasing the risk of exacerbations and their consequences, such as hospitalisations and absenteeism. In developed countries, more vulnerable members of society, such as children and minorities, are at higher risk from asthma.1
Asthma in Latin America
Latin America covers 13% of the Earth’s terrestrial surface and is home to approximately 600 million people. Despite being viewed as a homogenous cultural block, the region is highly diverse with a great breadth of sociodemographic characteristics, multiple languages and religions, and health services with disparate structures and resources. There is currently poor knowledge concerning asthma prevalence and precipitating factors within the region;2 however, the data that do exist indicate that asthma is a significant health problem in Latin America. In the 2009 phase of the global International Study of Asthma and Allergies in Childhood (ISAAC), Latin American countries often fell within the highest prevalence category (≥20%).3 In a recent systematic review, the prevalence ranged between 7% in Mexico and 33% in Peru (Figure 1).2
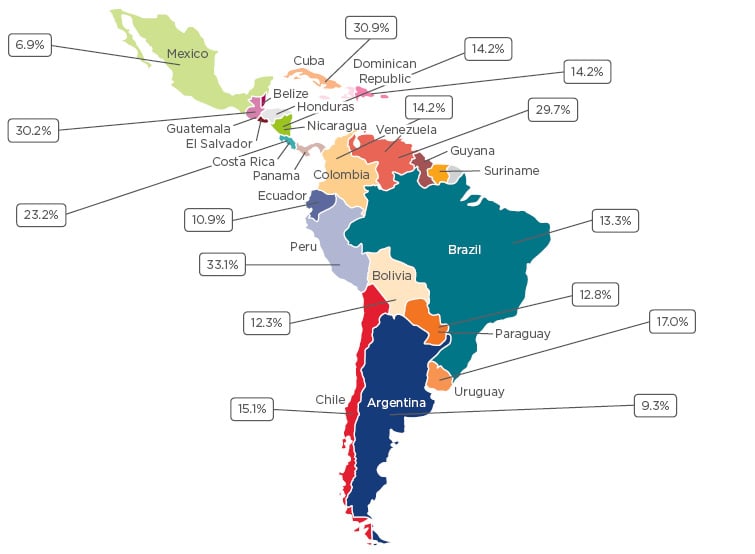
Figure 1: Asthma prevalence in Latin American countries.2
Natural History of Asthma and Wheezing
Severe asthma is associated with reduced quality of life, school and work absenteeism, and increased healthcare costs. Data from the longest running longitudinal cohort study on asthma, initiated in the 1970s, indicates that poor control in childhood translates into increased severity and morbidity in adulthood.4-7 Debate exists regarding whether increased asthma prevalence reflects a true epidemiological shift or whether it is driven by improved detection. The weight of evidence, however, suggests that the true global prevalence is increasing. Knowledge of risk factors has improved substantially over recent decades, allowing researchers to explore whether increased exposure may explain the increased prevalence of asthma. Risk factors may be region specific; for example, pollution and diet in Eastern Europe and obesity and stress in developed Western countries. The diversity of Latin America means that the full range of global risk factors can be found within the region.1,2
Exposure to asthma risk factors may begin prenatally with tobacco smoke and maternal diet. Diverse postnatal sensitising factors include exposure to mites, smoking, pollution, ozone, infection, and vitamin D deficiency.8 Latin American data suggest that societal factors, such as exposure to violence, may also increase asthma prevalence.8-10 Perhaps the three principal predisposing factors, however, are genetics, pollution, and early exposure to viral infections. These widespread risk factors share a common consequence: increased proinflammatory changes, which are the basis of asthma pathophysiology.8
Definitions, Genetics, Phenotypes, and Pathophysiology
Asthma is no longer considered a disease, but rather as a heterogeneous group of conditions that result in recurrent, reversible bronchial obstruction, characterised by inflammation, hyperresponsiveness of the airway, and airflow limitation.11,12 Defining a heterogeneous group of conditions poses a challenge. The classical definition of asthma characterises the condition in terms of recurrent episodes of shortness of breath, with cough and wheeze, occurring mainly at night. Using this definition, most patients have mild disease, risking under-diagnosis, under-treatment, and inadequate control. The physiological definition focusses on airflow limitation and the presence of inflammatory biomarkers. In Latin America, spirometry to detect airflow limitation is underutilised, particularly in children, and, in some cases, is not available. Finally, the pathological definition focusses on chronic inflammation, with or without airway changes, including remodelling.
A definition based on genotype is not simple, as >100 asthma-associated genes have been identified so far. Asthma also has multiple phenotypes, including episodic, exercise-induced, and atopy-induced asthma. Recently, definition via endotype, where disease subtype is classified by the distinct pathophysiological mechanism, has been proposed. The requirement to consider the influence of epigenetics on gene expression adds a further layer of complexity to this already multifactorial environment.
The wide range of heritability (35−95%) is in line with the multiple associated genes, endotypes, and mechanisms. Data suggest single nucleotide polymorphisms have a weak influence on asthma risk. Studies of common genetic variants suggest associations with viral infection or vitamin D deficiency mechanisms; however, so far, genetic studies have made little progress towards prognostic utility.11 The Latin American population appears to share a large overlap of candidate genes with other populations, including transforming growth factor beta 1 (TGF-β1), 17q21 locus, interleukin (IL)-13, glutathione S-transferase Mu 1 (GSTM1), matrix metalloprotein (MMP) 9, and β2 adrenoceptor (ADRβ2). Genes associated with asthma severity, vitamin D and E deficiency, and obesity, such as thymic stromal lymphopoietin and MMP12, were first identified in Latin America. Epigenetic studies have also identified interactions between candidate genes related to IL-10, TGF-β1, and dust mite allergens, and the previously mentioned association with exposure to violence is associated with DNA methylation of the ADCYAP1R1 gene.
The three classical phenotypes of transient early wheezers, non-atopic wheezers, and immunoglobulin (Ig)E-associated wheeze/asthma have been split into six pathophysiological wheezing endotypes, here presented in ascending order of wheeze persistence: never or infrequent, pre-school-onset remitting, mid-childhood-onset remitting, school age-onset persisting, late childhood-onset persisting, and continuous wheeze.13,14 In a longitudinal cohort study, the prevalence of these phenotypes was 60.0%, 19.0%, 7.5%, 4.3%, 4.7%, and 4.9%, respectively.14 The detailed characterisation of asthma into endotypes associated with features such as the extent of IgE and T helper (Th)2 involvement, relationship to obesity, IL-5 predominance, and relationships with steroid and β2 agonist response, poses a great challenge in the field.
Inflammatory processes, influenced by both genetics and environmental factors, are fundamental to the pathogenesis of asthma. More recent data suggest a central role for the airway epithelium in asthma. Pathogens, allergens, and pollutants activate innate signalling receptors in the airway epithelium, by pathogen-associated molecular patterns, damage-associated molecular patterns (also called alarmins), and allergens resulting in the release of three principal cytokines; IL-25, IL-33, and thymic stromal lymphopoietin.15,16 The resulting production of Type 2 cytokines, particularly IL-4, IL-5, and IL-13, results in increased trafficking of immature antigen-presenting dendritic cells to the epithelium, and increased antigen processing. These dendritic cells then drive T cell differentiation, often favouring Th2 cells, and consequent inflammatory processes.15
Bronchial biopsies of asthma patients reveal significant airway inflammation characterised by the presence of eosinophils, lymphocytes, neutrophils, and mast cells. Structural changes including smooth muscle hypertrophy and epithelial denudation also occur. Though still important, the previous position of eosinophils as the key asthma mediator has been superseded by the airway epithelium, particularly during exacerbations, and Th2 cells, which are definitive in determining whether asthma is atopic or non-atopic. The role of neutrophils and oxidative stress has also gained increased prominence in recent years.17,18 The recently published Mexican asthma treatment guidelines19 propose two main branches in the pathophysiology of asthma: the Th2/ILC2/NKT branch, which results in eosinophilic inflammation in an IgE-dependent (Th2) or independent (ILC2/NKT) fashion; and the Th1/ILC1/Th17/ILC3 branch, which drives neutrophilic inflammation via an interferon (INF)γ-dependent (Th1/ILC1) or an IL-17-dependent pathway (Th17/ILC3). Ultimately, both pathways result in the bronchospasm associated with asthma exacerbations.19
Tissue changes caused by asthma include infiltration of the above inflammatory cells, as well as mucus cell hyperplasia. Thickening of the subepithelial basement membrane increases in smooth muscle mass both through hypertrophy and hyperplasia, and the presence of mucous plugging are classical features of asthma.20 The median onset of asthma is 3 years in males and 8 years in females; in males, 80–90% of cases occur before the age of 4 years.21 Previously, airway remodelling was thought to take place only in chronic or severe cases, particularly in adults. We now know this is not the case, it occurs in children, and may be present regardless of level of severity. Data now suggest that pathological changes also begin at an early stage of disease. Increases in inflammatory cells and leukotrienes are evident in children aged <3 years with persistent wheezing.22 Basement membrane thickening and eosinophil infiltration appear to take place between the ages of 1 and 3 years, though they are absent in younger infants with persistent wheeze.23,24
The Role of Infection
Microbes represent a dichotomy for asthma risk. A balanced microbiome is essential for the development of the immune system and avoidance of atopy, while infectious pathogens increase the risk of developing asthma and suffering exacerbations.25
Hospitalisation due to respiratory syncytial virus (RSV) or rhinovirus-associated bronchiolitis during infancy are strongly associated with the presence of allergy and asthma later in life.26,27 Wheezing caused by rhinovirus during the first year of life is the strongest predictor of wheezing at 3 and 6 years of age, and severe RSV leading to wheezing in early life increased asthma prevalence to approximately 40% by 18 years of age, compared with 9% in controls.26,28-30
When comparing the two pathogens directly, wheezing related to rhinovirus (odds ratio [OR]: 9.8) in the first 3 years of life conferred significantly more risk of developing asthma by 6 years old than RSV only (OR: 2.5). Furthermore, the combination of rhinovirus and RSV (OR: 10.0) conferred only a marginally higher risk than rhinovirus alone. In total, 90% of children who experienced wheezing due to rhinovirus by 3 years of age had asthma at 6 years of age.31 This pattern continues into adolescence, with wheezing caused by rhinovirus in the first 3 years increasing risk of asthma, adjusted for wheeze caused by other infections, up to the age of 13 years, at which point RSV no longer increased risk (OR: 1.0). Early infection (<1-year-old) with rhinovirus also results in a greater risk of asthma during the first 5 years of life.32
In a long-term prospective study of patients with severe RSV bronchiolitis (N=206), 73% of children exhibited wheezing at 3 years old and 30% of patients had a diagnosis of asthma at 5 years of age. At 13 years old, RSV causing severe bronchiolitis resulted in an OR of 9.3 for the development of asthma or recurrent wheezing.29 In a Costa Rican study (unpublished data), similar patterns were observed. Children (N=172) hospitalised for bronchiolitis during the first year of life, 25% of whom were pre-term deliveries, were contacted at age 6–7 years. Almost 40% of the patients had been diagnosed with asthma, 73% had a history of wheeze, 3% had visited the emergency room (ER), and 50% had a history of nebulisation.
In a study investigating non-viral infections, hypopharyngeal bacterial colonisation in asymptomatic infants was associated with wheezing/asthma by age 5 years (OR: 4.5; 95% confidence interval [CI]: 2.18–9.57). In addition, the risk of hospitalisation was also higher for those colonised as neonates. Of the four bacteria investigated, the risk was increased for colonisation by Streptococcus pneumoniae, Haemophilus influenza, and Moraxella catarrhalis, but not Staphylococcus aureus.33
Prospective interventional data on palivizumab, a monoclonal antibody against RSV, further strengthen the causal link between viral infection and wheezing or asthma. Palivizumab is recommended for high-risk infants, such as premature babies, and has achieved an approximate 50% reduction in infection rates.34 The multicentre, matched, double cohort study investigated palivizumab asthma prevalence in premature infants. Preterm infants who were treated with palivizumab during the previous season and not hospitalised for RSV during the following winter (n=191) were matched with two control groups with (n=76) or without (n=154) a documented RSV hospitalisation. Palivizumab therapy resulted in a reduction in the relative risk of recurrent wheezing through the ages of 2−5 years in patients with no family history of atopy (80% reduction) or asthma (68% reduction).35 There was no reduction in patients with a family history of atopy, where patients are at a higher risk of developing asthma later in life. Similar randomised controlled trial results demonstrated a significant reduction in cumulative wheezing over the first 12 months of life in preterm infants treated with palivizumab.36 An article supports these data, suggesting that palivizumab administration in pre-term infants suppressed recurrent wheezing during the first 6 years of life, however, it did not suppress the onset of atopic asthma. Thus, morbidity is reduced despite there being no prevention of asthma development.37 These data are in line with the hypothesis that, in order to avoid the development of asthma, three arms of risk driven by allergy, viral infections, and the microbiome of the airways must be addressed, presenting a significant challenge for the healthcare system.38
The importance of the microbial environment in the development of asthma has been elegantly illustrated in two studies comparing asthma rates between children from the Amish and Hutterite communities. Though genetically very similar, exposure to endotoxins in dust was 6.8-times higher in Amish children, corresponding to a 4–6-times lower rate of sensitisation, compared with Hutterite children. Authors identified innate immune system exposure to a rich microbial environment as the source of the difference in allergy and asthma between the two communities.39,40
Asthma Diagnosis
Variability in the presentation of asthma and age-dependent changes in children’s symptom patterns add an extra layer of complexity to asthma diagnosis. A good clinical history, with knowledge of family background, risk factors, and previous infections is key, as is a thorough physical examination and differential diagnosis to rule out other conditions, such as the presence of foreign bodies or cystic fibrosis, or comorbidities, such as allergic rhinitis, sinusitis, and gastro-oesophageal reflux.
Rapid and accurate diagnosis is particularly important in infant and pre-school children due to a high prevalence of wheezing and associated morbidity. In this age group, 48% of children have experienced an exacerbation in the previous 12 months. Infants and pre-school children have the most severe episodes of exacerbations due to viral triggers, with worse symptoms and more frequent hospitalisations.41 However, heterogeneity, overlapping symptom profiles, and a lack of objective or practical measures make diagnosis particularly challenging. Common differential diagnoses include recurrent viral respiratory infections, gastro-oesophageal reflux, foreign body aspiration, bronchomalacia, cystic fibrosis, primary ciliary dyskinesia, and vascular ring, amongst others.
In children ≤5 years old, clinical history is fundamental as it can help determine if episodes are recurrent or persistent, or if they are related to exercise or triggered by exposure to environmental pollutants. These details may help diagnose the endotype of asthma present. Family history of allergic disease should be considered, particularly in first-degree relatives. Finally, clinical improvement during 2−3 months of treatment and worsening following withdrawal are indicative of asthma.
Treatment Guidelines
Multiple treatment guidelines for asthma exist, however, broadly their messages are in sync. The recently published Mexican asthma treatment guidelines integrate aspects of UK, Global Initiative for Asthma (GINA), Spanish, and Australian guidelines, adapting them from the highly varied regional nature of Mexico.19 Goals include adequate control of the symptoms and exercise tolerance, reducing exacerbations, avoidance of side effects, and maintenance of normal lung function. Regional and cultural factors affect tactics for asthma treatment which should be tailored to individual patient needs, in relation to cultural or ethnic beliefs and practices. The goals of treatment remain the same regardless of the societal or economic factors.
The recommended stepwise approach to treatment in children, especially in children <5 years, begins with the gold standard low dose daily inhaled steroids. The majority of patients will respond to this without the need to escalate to a low double dose or specialist assessment. Unfortunately, current treatment paradigms do not modify the evolution of the disease and withdrawal of treatment will result in manifestation of symptoms and pulmonary function, similar to an untreated individual at the same disease stage.42
Several components are needed to achieve asthma control. First, the most appropriate treatment must be chosen for the patient. In the majority of patients with mild asthma, adequate treatments exist to achieve disease control; however, other factors need to be addressed that may impinge on the ability of the patient to control their asthma. Economic difficulties may impede access to treatment, lack of parental education may affect aspects of control, spacers may be needed and should be provided where necessary, lack of adherence (~70%) must be addressed, and risk factors or triggers such as smoking should be reduced as much as possible. The above measure will also be useful in the 5% of patients with severe asthma who are at greatest risk of exacerbations.
Managing Wheezing and Asthma Exacerbations
Control of asthma means different things to different people. From the patient’s perspective, control may mean the ability to live a full life, attend work or school, take part in activities, and achieve undisturbed sleep. The physician will often focus on long-term outcomes, avoiding overuse of steroids, for example, due to long-term effects on bone density. From the perspective of the healthcare system as a whole, reducing cost and disease burden are the Holy Grail. Happily, maintaining physiological control and avoiding exacerbations can achieve all these goals in the long term.
Current Control
A logarithmic relationship exists between lower Asthma Control Test scores, indicating worse control, and higher odds of exacerbations.43 Patients with partially controlled and uncontrolled asthma are, respectively, two (OR: 1.97; 95% CI: 1.53–2.54) and six-times (OR: 5.74; 95% CI: 4.52–7.29) more likely to suffer exacerbations compared with patients with full control.44
Data from 2005 suggest asthma control in Latin America was inadequate during this period. The large Asthma Insights and Reality in Latin America (AIRLA) study45 (N=2184) surveyed adults with asthma and parents of affected children about healthcare utilisation, symptom severity, activity limitations, and medication use. Almost 50% of adults and children had symptoms every day and night. Symptoms during exercise were common (40%) and the majority of adults reported their ability to exercise was limited. Disruption to sleep and limitation of daily activities were also common. The majority of both adults and children had required an ER visit, with close to three-quarters of children requiring emergency treatment. ER visits were more common in children, likely due to the higher rate of viral infection in this age group. Strikingly, <3% of the patients met GINA criteria from asthma control. An additional cause for concern was the underutilisation of inhaled corticosteroids across all severity groups (mean: 6%), reflecting a general non-adherence to guidelines. Patient perceptions regarding asthma control were far from in line with reality in the AIRLA study. Forty five percent of those surveyed perceived their asthma as well controlled compared with the 2% controlled according to GINA criteria.45 All data is displayed in Figure 2.
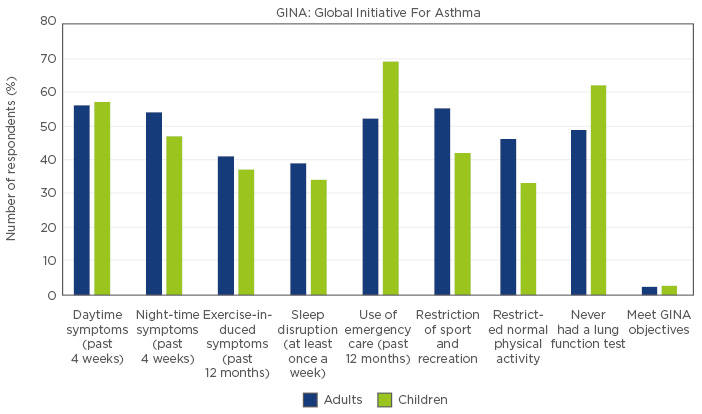
Figure 2: Indices of asthma control in Latin America from the AIRLA study.45
In asthma, as in all chronic disease, the risk of disease development accrues over time with cumulative exposure to risk factors. However, data from the long-term Melbourne asthma study (MESCA), 1964−2007, indicate that despite this long-term accumulation, the greatest single predictive factor for severe asthma at the age of 50 years is severe asthma during childhood.7 Therefore, the large number of children from the AIRLA study with an occult lack of control due to family misperceptions represent a high risk for future severe illness in the population (Figure 2). Furthermore, recent data indicate there is a significant phenotypic overlap between asthma and chronic obstructive pulmonary disease, and both children and adults with impaired lung function are at increased risk of developing fixed airway obstruction, and possibly chronic obstructive pulmonary disease in early adulthood.46
In Finland, a national programme reduced asthma morbidity and its impact on both society and individuals, illustrating the ability to affect long-term positive change.47 Similar improvements have been achieved in Latin America, where implementation of guidelines based on GINA saw a steady fall in hospitalisations from 1997 onwards. The introduction of affordable corticosteroid care with beclomethasone and its adoption by general practitioners had a further positive impact. Between 1997 and 2011, hospitalisation of children and adolescents reduced by 50−60%, and reductions in adults were 50−74%. There were also marked reductions in mortality in all age groups over this period, evidently related to increased physician awareness and improved prescribing patterns.48
Exacerbations and Their Triggers
Whether asthma is well controlled or not, exacerbations tend to be associated with triggering factors. Among various possible factors, viral infection is the most important, particularly in younger children. Approximately 85% of children who experience an acute asthma attack have a viral infection at the time. Among children hospitalised for wheezing, RSV, influenza virus, and rhinovirus are most common in those <3 years old, and rhinovirus is most common in children >3 years.49 Other risk factors in children <5 years old include uncontrolled asthma symptoms, ≥1 severe exacerbation in the preceding 12 months, season, tobacco smoke, indoor or outdoor air pollution, indoor allergens, psychological or socioeconomic problems, poor adherence or incorrect spacer or inhaler technique, exercise, cold air, and medications.
In children <2 years of age, viral wheezing is often associated with risk factors including tobacco smoke exposure, reduced lung function, and a lack of breastfeeding. In older children, viral wheezing is often associated with elevated IgE, inhaled allergen sensitisation, and maternal asthma. In a Costa Rican study,50 children with uncontrolled asthma admitted to the ER with an exacerbation were studied and rhinovirus was identified as the major causal agent. Allergic sensitisation to dust mites, demonstrated by high IgE titres, was a significant risk factor for rhinovirus-associated wheeze in these patients (OR: 31.5; 95% CI: 8.3–108.0). These data are in line with a contemporaneous study showing that children sensitised to aeroallergens had a greater risk of developing viral wheeze (hazard ratio: 1.9; 95% CI: 1.2–3.1).51
Parasitic infection with Ascaris lumbricoides is a region specific environmental risk factor for asthma exacerbations in Costa Rica. Patients infected with A. lumbricoides displayed eosinophilia, decreased lung function, and increased airway hyper-responsiveness, alongside increased risk of hospitalisation for asthma (OR: 3.08, 95% CI: 1.2–7.7).52 Air pollution is another particularly pressing issue within the Latin American region and one likely to deteriorate due to climate change.53-55 Indoor pollution is associated with increased asthma symptoms and use of rescue medication. The issue is particularly prevalent in association with poverty and the use of combustible fuel to cook indoors.56 Unsurprisingly, data from meta-analyses indicate that parental and passive smoking increase the risk of wheezing (OR: 1.41) and asthma (OR: 1.85) in children <2 years old and the risk of developing asthma in children of ages 5−18 years (OR: 1.23).57
The impact of diet and vitamin D deficiency has also been investigated in Costa Rican children with asthma (N=616). The 28% of children with levels of vitamin D below guideline recommendations had the highest levels of IgE and eosinophils, more visits to the ER and hospitalisations, more anti-inflammatory treatment, and more bronchial hyperactivity.58
As previously noted, poverty is associated with risk factors for asthma exacerbations. The cycle of poverty drives people towards disease and impedes their ability to control it. Poverty limits socioeconomic opportunities resulting in risky behaviour and increased exposure to risk factors for asthma. When disease has taken hold, limited access to healthcare compounds the likelihood of exacerbations which may, in turn, lead to work absences and increased poverty, further compounding the situation.
Exacerbation Management
Asthma exacerbations are a medical emergency leading to a dramatic short-term decrease in lung function followed by a return below the previous baseline level. The result is a progressive deterioration in lung function, which is more marked in severe cases. Their main clinical feature is reduced expired airflow which may be measured by wheezing, progressive dyspnoea, hyperventilation leading to hypoventilation, and by testing pulmonary function (forced expiratory volume in the first second).59,60 Factors increasing the likelihood of death from an asthma exacerbation include a previous near-fatal exacerbation, previous intubation, hospitalisation in the previous year, >2 previous ER visits, use of oral corticosteroids or short-acting β2 agonists for control, and psychosocial issues.61
Pathophysiologically, three conditions occur during an exacerbation: bronchoconstriction, inflammation, and hypoxaemia. These are corrected with early use of short-acting β2 agonists, steroids, and oxygen, respectively. Nebulisers are no longer recommended for the management of asthma due to increased application and waiting time, higher cost, and lack of portability. Inhalers or spacers, with education for proper use, have the advantage of a lower application time with no wait, lower cost, and portability. Children in crisis treated with a salbutamol inhaler at home are likely to arrive at hospital in a significantly better state than those not treated at home. However, spacers must be the appropriate size for the patient, allow rapid inhalation of the corticosteroid, and be applied in a manner that does not further restrict breathing.
The majority of treatment guidelines in Latin America and worldwide follow a similar path to those recommended by GINA. The first stage is examination to determine if the exacerbation is mild, moderate, or severe/life-threatening. In a mild or moderate crisis, the patient should be started on short-acting β2 agonists immediately, plus oxygen if needed. Steroids are not recommended unless the crisis is severe requiring hospitalisation, due to deleterious effects on bone development. Magnesium sulphate may be used as a second- line medication for patients >12 years of age who are experiencing a severe crisis. Following improvement, a short-acting β2 agonist should be continued during the observation period and the opportunity taken to educate the patient or parent regarding use of inhalers or spacers, risk factors, and other factors which may improve control.
Two aspects of treatment are often overlooked: time for recovery and prevention. Prevention can be aimed at better use of medication including proper use of inhalers, adherence to treatment, side effects, use of spacers, costs, and availability. Non-medication-related prevention covers education regarding risk factors, especially respiratory infections through washing hands and avoiding contact with sick people, reducing indoor and outdoor pollution, and improving nutrition and exercise.
The medical community has effective medications, effective evidence-based guidelines, and an active research community collaborating at an international level. However, it lacks resources, time to educate patients, proper compliance from patients, control of disease, and risk factors, local cohort studies and, most fundamentally, a cure. Progress towards these unmet needs is required to meet the medical community’s responsibilities to patients.
Immunomodulators: Role in the Prevention of Asthma Development and Exacerbations?
Multiple precipitating factors may initiate an asthma exacerbation, from temperature change to allergens, pollutants, stress, exercise, infection, or smoking. Preventative measures, such as the use of immunomodulators, may reduce the immune system’s responsiveness to these triggers and thus reduce the extent of the exacerbation.
Currently, there are promising results from five studies of the bacterial lysate immunomodulator OM-85 for the prevention of wheezing or asthma in children. In a small randomised single-centre study conducted in China, infants hospitalised with wheezing, who required oxygen support, glucocorticoid, or bronchodilator treatment were randomised to receive post-discharge OM-85 (n=24) (standard dosing: 3.5 mg/day for 10 days/month for 3 months) or standard dosing or budesonide aerosol 200 µg once or twice daily (n=19). A group of untreated healthy controls was also included in the study (n=10). During the 1-year follow-up, there was a 38% reduction in the risk of recurrent wheezing. Only 25% of the infants suffered recurrent wheeze after receiving OM-85 compared with 63% receiving inhaled steroids (p<0.05).62
A double-blind, randomised, placebo-controlled, parallel-group study on OM-85 in patients aged 1−6 years, with virus-induced recurrent wheezing was carried out in Turkey. Seventy-five children received OM-85 or placebo (standard dosing) with a 1-year follow-up. OM-85 reduced the number of wheezing attacks by 38% over 12 months (Figure 3) (mean difference: 2.18; 95% CI: 3.22–1.13; p<0.001) and the difference was highly significant from 9 months onwards (p=0.001). In line with the relationship between infection and wheezing events, the number of acute respiratory tract infections (RTI) (-2.44 [-3.5 to -1.36]; p<0.001) and episodes of nasopharyngitis (-2.11 [-2.94 to -1.27]; p<0.001) was reduced over the 12 months, with a highly significant reduction from 6 months onwards for both conditions (p<0.001). Patients treated with OM-85 also achieved a reduction in the cumulative number of wheezing days per patient and duration of wheezing attack (p<0.001). The authors concluded that OM-85 was a useful complementary treatment to reduce the number and duration of RTI-induced wheezing attacks in pre-school children.63 It is worth keeping in mind the phenotypic differences in wheeze in patients between the ages of 1 and 6 years, which may have affected the results.
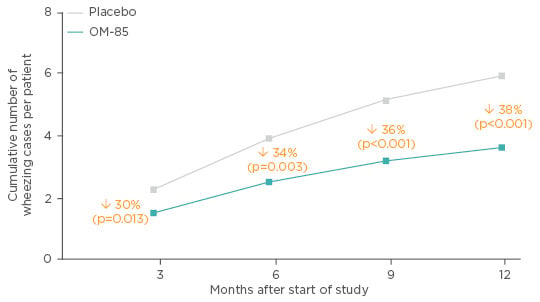
Figure 3: Effect of OM-85 prophylaxis in pre-school children with a history of recurrent viral wheeze.
Adapted from Razi et al.63
A 1-year, single-centre, prospective, open-label study compared OM-85 (n=29) to inhaled corticosteroids (n=16) in school children with acute stage asthma. The choice of study arm was left to the participants, introducing a selection bias. OM-85 reduced asthma exacerbations (55% reduction) and respiratory infections (61% reduction). There was a significant corrective effect on the Th1/Th2 cytokine (INF-γ:IL-4) imbalance in both patient groups. Lung function was also improved in the OM-85 group compared with ICS treated patients; however, these results should be interpreted with caution as they were not adjusted for height, sex, or age.64
A prospective, randomised, double-blind study compared OM-85 (n=35) prophylaxis or placebo (n=40) in children aged 1−12 years old with asthma and recurrent RTI. The active arm received standard dosing of OM-85 in Months 1–3, and again in Months 7–9. There were marked reductions in the number of upper and lower RTI (URTI and LRTI) at 6 and 12 months versus baseline (p<0.05) and versus placebo (p<0.01) (Figure 4). Furthermore, there were significant increases in serum human β-defensin-1, IgA, and IgG in the treatment group compared with baseline at Month 6 and 12 (p<0.05) and compared with the placebo group at Month 6 and 12 (p<0.05).65
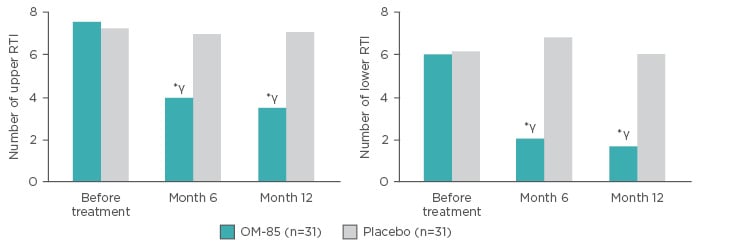
Figure 4: Number of upper respiratory tract infections and lower respiratory tract infections in patients receiving OM-85 prophylaxis or placebo.
*p<0.01 (versus placebo); γp<0.05 (versus before treatment).
RTI: respiratory tract infection.
Adapted from Liao and Zhang65
A recent randomised trial compared OM-85 in combination with standard of care (n=74) to standard of care alone (n=62) in infants with viral capillary bronchitis and secondary bronchial asthma. Addition of OM-85 to standard of care resulted in significant improvements in capillary bronchitis and asthma at 1-year post-treatment (Figure 5). In addition, there were improvements in IL-17, IL-4, IL-10, and INF-γ levels in the OM-85 group.66
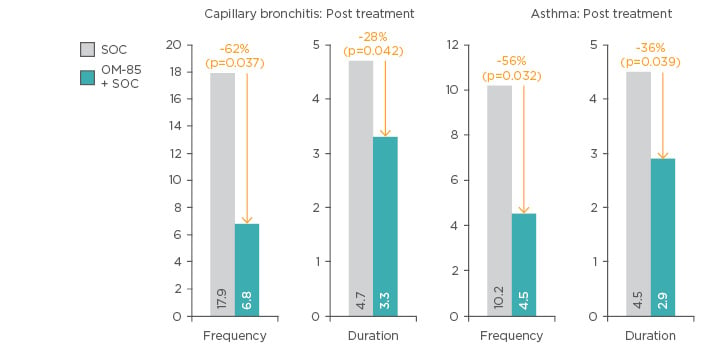
Figure 5: Frequency of capillary bronchitis and asthma exacerbations following addition of OM-85 to standard of care versus standard of care alone.
SOC: Standard of care.
Adapted from Han et al.66
Patients at Risk
At-risk patient groups for wheezing who may be considered for OM-85 prophylaxis include pre-term infants, children <6 months of age, children who were never breast-fed, patients with an underlying heart or lung condition (e.g., cystic fibrosis and Down’s syndrome), children with a depressed immune system, children exposed to tobacco smoke, and children exposed to crowded environments (e.g., day-care and siblings). As physiological vulnerability decreases with age, exposure increases, keeping the risk for severe bronchitis or wheezing high.
Hospitalisation due to RSV infection is substantially more common in premature babies (6−7%) compared with healthy controls (2−3%). In infants with chronic lung disease, hospitalisation rates approaching 39% may be observed during the first year of life.67 Children with Down’s syndrome are at very high risk of hospitalisation due to alterations in their lung structure. The prevalence of hospitalisation for this group is approximately 10% during the first 2 years of life.68
At-risk groups for persistent asthma include those with a parental history of asthma (especially maternal) and allergy; those with eczema or allergic rhinitis; those experiencing persistent wheezing, wheeze without viral infection, or exercise-induced wheeze; those experiencing severe wheezing episodes; patients with allergic sensitisation, especially early poly-sensitisation; and those with increased numbers of eosinophils and fractional exhaled nitric oxide.
OM-85: Future Perspectives and Research
Despite almost 35 years of clinical use, there are still many pending questions regarding the mechanism of OM-85. Currently there are no data examining which of the 21 strains of bacteria contained in OM-85 are essential for its immunomodulatory effect, or if the presence of all the strains is required. The bacterial components and the specific pattern-recognition receptors, likely toll-like receptors, responding to them have yet to be identified. Studies comparing different doses or dosing schedules have not been carried out, and sufficient data on whether activity is equal in patients with allergic and non-allergic wheezing are currently unavailable.
There are currently four ongoing studies investigating OM-85 in wheezing and asthma that may address some of the above questions. The OMPAC study69 is currently underway in Australia, including 60 infants aged 3−9 months at risk due to a sibling with asthma or atopy. This randomised trial will compare outcomes in infants treated with OM-85 in two cycles during the infants first two winter seasons (3.5 mg; 10 doses/ 5 consecutive months) versus placebo. The study will compare primary prevention of symptomatic LRTI, persistent asthma development, nasal microbiota, circulating T-regulator cells, allergen sensitisation, and transcriptomics analysis. Results for OMPAC are expected in June 2019.
The ORBEX study70 is a large, multicentre, randomised, placebo-controlled trial, with upwards of 8 centres in the USA (N=1,076). The objective is to determine if OM-85 (3.5 mg/day for 10 days/ month for 2 years) given to infants (6-18 months old) at high risk due to having atopic eczema and/or having a parent or sibling with asthma can increase the time to occurrence of the first episode of wheezing, due to a lower respiratory tract illness during a third observational year after therapy.
Exclusion criteria include more than one previous serious episode of wheezing or more than two mild episodes. Severe wheezing is defined as cough and wheezing >24 hours and one of the following: >6 doses of albuterol in <48 hours, ER visit or hospitalisation, use of inhaled or systemic corticosteroids, diagnosed asthma, or a systemic illness (other than allergy). The primary outcome is time to first wheezing episode during follow-up. Secondary outcomes include wheezing rate during treatment and during follow-up, time to first wheezing episode during treatment, and the rate of severe wheezing during treatment. Adverse events will be assessed, and DNA, serum, and stool samples will be collected for future genetic studies assessing the bacterial and viral flora. Recruitment will run from January 2017 to December 2018, with preliminary results expected by December 2021.70
The Italian OMPeR trial will investigate OM-85 for the prevention of URTIs in children aged 1−5 years at high risk due to mild immunodeficiency (IgA, IgG), atopy, or recurrent wheezing. Children will be randomised to receive OM-85 at standard dosing or placebo, stratified by risk factor. An additional exploratory arm will investigate longer term dosing of 10 days for 6 consecutive months. Prevention of URTI during a 6-month follow-up will be investigated alongside URTI duration and severity, mean number of LRTI, RTI requiring antibiotics, bacterial tonsillitis, acute otitis media, school days lost, antibiotic therapy and cycles, and faecal microbiota. For patients recruited during an active infection at the first visit, time to cure will also be measured. Results are expected in 2018 (Prof S. Esposito, personal communication, 2017).
Finally, the BREATHE study71 has an alternative goal of improving asthma control. Adolescents and young adults (12−40 years old; N=120) with uncontrolled asthma (GINA 4 and ≥2 asthma exacerbations during previous winter season, Asthma Control Questionnaire >1.5) will be randomised to receive either OM-85 at 7 mg/day for 10 consecutive days during the October−March viral season for two consecutive seasons, or placebo. Following the 18-month treatment period, spanning two winters, there will be a 1-year follow-up. Reduction of asthma exacerbations, including suspected infectious exacerbations, will be assessed alongside nasopharyngeal/faecal microbiome and inflammatory markers in serum or sputa. Results are expected in 2020.71
The Next Steps
Two previously mentioned at-risk groups, pre-term infants and infants with Down’s syndrome, offer opportunities for research into putative unmet clinical need for OM-85 prophylaxis. The scale of this unmet need in Latin America is illustrated by a Brazilian longitudinal birth cohort study in pre-term infants (N=310), BREVI, which showed extremely high rates of LRTI (58%) and severe LRTI (21%), 61% of which were associated with RSV.72 Given the large number of infants hospitalised for RSV alone (n=56), the investigation of the putative ability of OM-85 to reduce hospitalisations in this population should be a priority.
CONCLUSION
The prevalence of asthma is increasing worldwide, and is associated with substantial economic and humanitarian effects. Viral infections are important in the development and exacerbation of asthma, alongside a complex interaction between genes and the environment. RTI cannot be avoided completely and contact with micro-organisms is a necessity of immune system maturation. However, immunomodulators offer a dual protective mechanism via creating pre-alert immune state which reduces infection rate and by reducing excess inflammatory processes. The immunomodulator OM-85 reduces URTI, LRTI, and wheezing in children and studies are underway investigating the effect of OM-85 on the prevention of asthma in high-risk children.








