Meeting Summary
Currently, there are many different inhaler options for the treatment of airway diseases. While having options can be of benefit, it also presents a challenge for physicians and other healthcare providers (HCP). Selecting the correct drug and dose is only one-half of their role; they also need to be sufficiently familiar with each device to be able to select the best option for each patient, as well as correctly advising patients on their use, as errors with inhaler use adversely affect the success of treatment. Understanding the key principles in device technology will help clinicians to navigate this field.
In the first presentation, Prof Rabe considered the selection of treatment in patients with chronic obstructive pulmonary disease (COPD) and discussed which patients are most likely to benefit from the use of inhaled corticosteroid (ICS). He noted that the largest effect size in exacerbation reduction was achieved in patients with higher levels of blood eosinophilic component and/or a past history of asthma.
Prof Lehtimäki then provided an overview of inhaler device technology, environmental factors relating to their manufacture and disposal, and the application of digital technology to improve inhaler use and patient adherence. Because of the high greenhouse gas potential of propellants in pressurised metered-dose inhalers (pMDI), their carbon footprint is estimated to be 10–37 times higher than that of dry powder inhalers (DPI). Digital solutions will help to assess patient adherence and inhalation quality in the future.
Next, Dr Usmani discussed inhaler device handling errors and how to avoid them. During the last 40 years, handling errors have not decreased, and many HCP are thought to be overwhelmed by the plethora of devices and drugs on offer. Education in the correct use of devices for both trainers and users is paramount to the success of inhalation therapy. Important advice includes avoiding the prescription of different devices to the same patient, and to favour combination products to avoid multiple inhalers.
Finally, Prof Bateman reviewed the rationale and evidence for maintenance and reliever therapy, and in particular the value of the anti-inflammatory effect of ICS doses taken when symptoms occur. This approach, when compared with similar or even higher maintenance doses of ICS with a short-acting β-agonist (SABA) reliever, has been consistently associated with lower exacerbations rates.
Why Do Some Patients with Chronic Obstructive Pulmonary Disease Benefit from Inhaled Corticosteroid Treatment?
Professor Klaus Rabe
Prof Rabe first showed the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) initial treatment guidelines and explained the reasoning behind the recommendation to consider ICS treatment for patients with a blood eosinophil count >300 cells/µL. An analysis by Harries et al.1 summarised the effect of ICS on COPD in 12 clinical studies. While the overall effect size was positive, there were a few outliers. It transpired that the higher the eosinophil threshold selected in each trial, the larger the effect size; this explained the difference between the studies. The conclusion was also supported by clinical trials on triple combinations, in which patients with a blood eosinophil count of >300 cells/µL were most likely to benefit from the addition of ICS.2-6 Prof Rabe concluded that ICS treatment must be personalised and based on clinical and biological markers. Markers strongly supporting inclusion of ICS in treatment are two or more hospitalisations for exacerbations per year despite appropriate long-acting bronchodilator therapy, a blood eosinophil count >300 cells/µL, and history or current diagnosis of asthma. ICS treatment may also be considered in patients with only one moderate exacerbation per year and a blood eosinophil count of 100–200 cells/µL. However, it should be avoided in patients experiencing repeated pneumonia events, those with a blood eosinophil count <100 cells/µL, and those with a history of mycobacterial infections.7
Key Features of Inhaler Device Technology
Professor Lauri Lehtimäki
Prof Lehtimäki then led the discussion on the relevance of inhaler technology for patients. Particle size and inhalation flow are the two factors that affect particle deposition within the respiratory system. Smaller particles are more likely to travel deeper into the lungs as they more easily follow the streamlines of the inhalation flow. However, particles that are too small are ineffective as they are not deposited within the time of inhalation and are exhaled. With fixed particle size, a higher flow rate leads to more central deposition than lower flow rates.8 In real life, all device-formulation combinations are optimised for specific inhalation manoeuvres and patients should follow the manufacturer’s instructions.
There are three main types of portable inhaler devices. DPI come in prefilled and capsule-based variants and rely on the patient’s inspiratory effort to disaggregate the drug particles from large lactose carriers. In general, the higher the resistance of the inhaler’s flow channel, the lower the required inhalation flow is. With the medium-high and high-resistance inhalers, the required inhalation flow is generally only 30 L/min.9 The benefit of using inhalation flow for drug dispersion is that the patient can prepare the inhaler for actuation and then inhale when they are ready, without the need to co-ordinate these events. In pMDI, propellant gas is utilised to aerosolise the drug solution or suspension. They require the patient to co-ordinate the actuation and inhalation for successful drug delivery. Spacers are often needed for optimal dosing, but different brands have been shown to affect dosing differently.10 There are also some models that are breath actuated. Soft mist inhalers create small droplets from solution via a mechanical apparatus rather than pressurised gas. There are many factors to consider when designing an inhaler, but the ideal inhaler device should be:
- effective
- efficient
- engaging
- error-tolerant
- easy-to-teach
- easy-to-switch-to
- environmentally friendly.
Prof Lehtimäki provided some practical tips on how to choose inhaled medication for the patient. The first was to define the pharmacological interventions required and to choose the drug class accordingly. Next was to discuss, try, and confirm with the patients the inhaler they are able to use and most comfortable with. Using more than one different type of inhaler increases the risk of inhaler errors;11,12 therefore, it is best to select a device type that can offer as many of the drugs needed as possible. It is paramount to always teach and check inhaler technique.
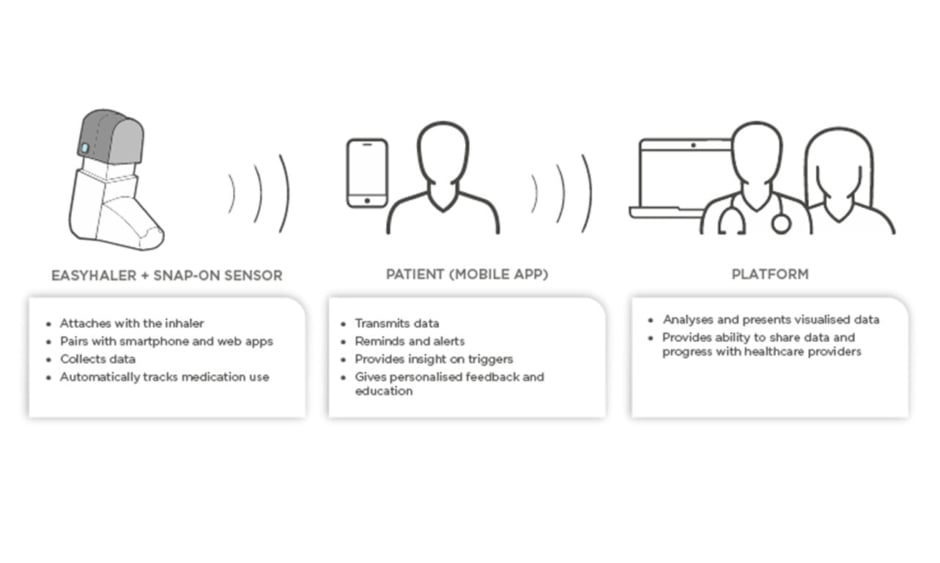
Novel devices with digital integration or add-ons are now entering the market. In the future, they will help clinicians to assess whether poor treatment results are attributable to poor adherence or lack of efficacy, as well as help the patient to play a more active role in their own treatment. The ideal digital aide should remind the patient to take their medication, monitor inhalations, and check the patient’s inhalation technique. As an example, Figure 1 shows the components of the Easyhaler® (Orion Pharma, Espoo, Finland) DPI connected to the Propeller sensor and platform. The sensor attaches to all Easyhaler products and pairs with the Propeller mobile app.
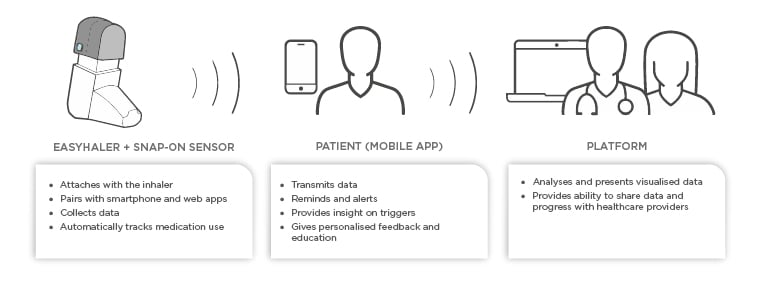
Figure 1: Schematic presentation of digital aide integration to mobile platform.
The platform provides the patients and caregivers with an opportunity to monitor adherence and to receive reminders when it is time to take a dose. The platform also provides personalised feedback and insight on triggers. Finally, HCP can access a portal to view patient data and ultimately make better informed therapeutic decisions. The use of such digital platforms has been shown to improve both patient adherence and asthma control.13,14
Patients, prescribers, and manufacturers are increasingly aware of the environmental impact of inhalation therapies. There are large variations between different inhaler devices in this respect. According to Montreal Protocol Medical and Chemicals Technical Options Committee (MCTOC) 2018 Assessment Report,15 pMDI have a 10–37 times higher carbon footprint compared to DPI. This conclusion was supported by an analysis by Janson et al.16 for selected marketed inhalers.15 Changing from a pMDI to DPI has almost the same impact on personal carbon footprint as changing to a plant-based diet or switching from gasoline to a hybrid car. As an example, breakdown of carbon footprint of Easyhaler DPI is provided in Figure 2.17 The difference between DPI and pMDI arises largely from the propellants used in pMDI devices. While original ozone-depleting chlorofluorocarbons have been phased out, modern hydrofluoroalkane propellants are still extremely powerful greenhouse gases.8 The patient’s ability to use the inhaler, their preferences, and efficacy of the medication are the most important factors in selecting inhaled medication, but environmental aspects of the device are emerging as a factor in treatment selection.
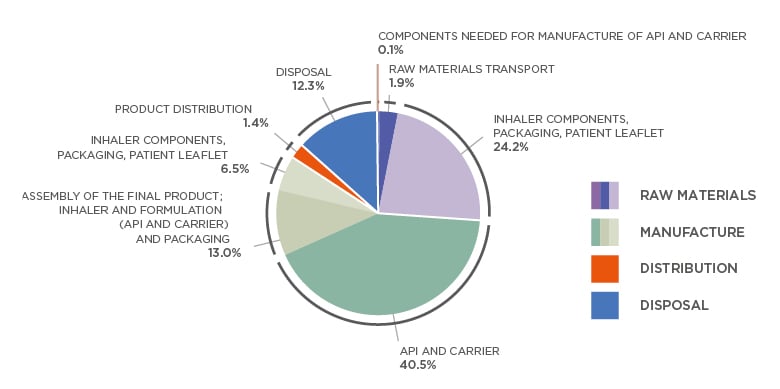
Figure 2: Breakdown of Easyhaler® (Orion Pharma, Espoo, Finland) carbon footprint according to raw materials, manufacture, distribution, and disposal.
Total cradle-to-grave life cycle emissions for one Easyhaler (average) is 0.588 kg CO2e.
Average of the four Easyhaler products (salbutamol, formoterol, salmeterol-fluticasone, and budesonide-formoterol Easyhaler). For each, the most used strength and number of doses per product was used for this analysis. Analyses were performed in accordance with ISO 14040 and ISO 14044 and verified by external carbon footprinting company (Carbon Footprint Ltd., Basingstoke, UK).
API: active pharmaceutical ingredient.
Adapted from Orion.17
Trainers and Trainees: How to Ensure Successful Inhalation Treatment for Patients?
Doctor Omar Usmani
Dr Usmani began his talk with an overview on inhaler development. However, the technological advances have not translated to more proficient use. A systematic review by Sanchis et al.18 showed that despite the advances in technology, the correct inhaler technique by the patient has been stagnated at approximately 30% for the past 40 years. However, the lack of inhaler knowledge is not only limited to the patients, but is also evident in HCP.19 Plaza et al.20 studied the inhaler technique of physicians, respiratory therapists, and pharmacists and found the success rate of HCP to be only 12%. Even though the inhaler device is essential to the treatment of pulmonary patients, respiratory doctors get very little or no training in the devices. Still, both the Global Initiative for Asthma (GINA) and GOLD treatment guidelines stress the importance of inhaler technique and adherence in the assessment of the patient.21,22 While it is generally agreed that inhaler errors are a problem, there is no consensus on the definition, which has been illustrated in a systematic review by Usmani et al.23 that found 299 descriptions of inhaler errors.
Different types of inhalers vary in how challenging the required inhalation manoeuvre is. Figure 3 shows results of a study conducted in the UK on patients with asthma in a primary care setting.24 Over 90% of the patients were able to achieve sufficient flow rates even for high-resistance inhalers, but >30% of the patients who used a pMDI were inhaling too forcefully. In conclusion, almost everyone was able to use a high-resistance DPI, but a significant portion of the patients using pMDI were not able to use their inhaler correctly, even after teaching and coaching by HCP.24
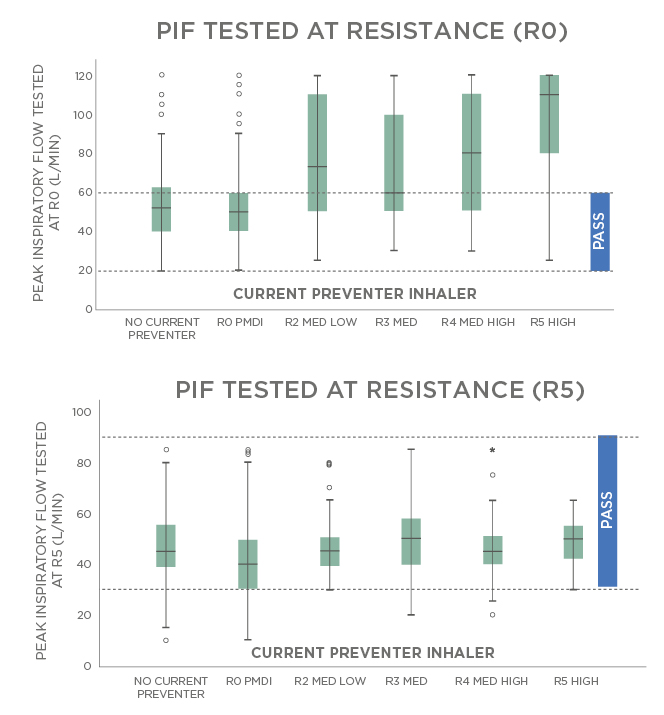
Figure 3: Measured inspiratory flows with pressurised metered dose inhalers and high dry powder inhaler resistances.
MED: medium; PIF: peak inspiratory flow; pMDI: pressurised metred dose inhalers.
Adapted from Haughney et al.24
British inhaler treatment guidelines promote the ‘ACT’-method for device selection.25 Firstly, when prescribing inhaled medications, the HCP should Assess whether the patient is able to inhale quickly, deeply, and forcefully (DPI) or slowly and steadily (pMDI, soft mist inhalers, or breath-actuated inhaler). The inhaler type that is better suited for the patient is then Chosen. Inhalers have considerable environmental impact and when it does not compromise the treatment, it should be considered when choosing the device for the patient. Lastly, the patient should be Trained to use their device with placebo inhalers and materials should be given so the patient can revise the technique. It is also essential to check and correct the patient’s inhalation technique at every opportunity.
A patient’s inhalation technique can be estimated using seven steps:
1. Preparation
- Check dose counter
- Shake inhaler if instructed
2. Priming
- Prime the device ready for use
3. Exhaling
- Exhale gently away from the mouthpiece
4. Mouth
- Place the mouthpiece in mouth, tilt the chin, and close lips tightly around the mouthpiece
5. Inhalation
- DPI: quick and deep
- pMDI: slow and steady
6. Breath holding
- Remove inhaler from mouth and hold breath for up to 5 seconds
7. Closing and repeating
- Close the inhaler/cap
- Repeat if necessary
Finally, Dr Usmani gave some practical tips for the clinicians. Firstly, the inhaler device should not be changed without talking to the patient. Doyle et al.26 showed that switching without consent may diminish self-control, which is associated with better asthma management. Secondly, inhaler devices should not be mixed. For patients with asthma or COPD it has been shown that the disease control worsens and exacerbations are more common in patients with inhalers needing different inhalation techniques.11,12 At least, the inhaler selection should be stratified to the same type of device. Thirdly, patients’ inhaler technique should be checked at every opportunity and fourthly, HCP need to train themselves. The most expensive inhaler is the one not used, or taught, correctly.
Changing Perceptions and Improving Asthma Outcomes: Spotlight on Maintenance and Reliever Therapy
Professor Eric Bateman
Prof Bateman described patient factors that influence adherence to ICS treatment in asthma.27 Paramount among these were the patient’s beliefs about the necessity for treatment and concerns about the safety of the treatment. Acceptance of the necessity for treatment with low concern about safety was associated with the highest adherence rates, whereas indifference or scepticism with or without concerns about safety led to low adherence. Additional factors for some patients are perceived social stigma or embarrassment about inhaler use, and fear of addiction.28 In a large European survey of patient attitudes to asthma treatment, fewer than one-half of the patients reported taking their inhaler every day, and most reported taking it on some days and not on others, or only when symptoms occurred.29 The problem of nonadherence with controller treatment results in many patients relying entirely on SABA taken as-needed, living with high levels of symptoms, and at risk of asthma attacks.30 In addition, regular (four times daily) SABA use has been shown to increase airway hyperresponsiveness and worsen eosinophilic inflammation compared to regimens that include an ICS.31 In national surveys, the prescription of three or more canisters of SABA per year has been associated with increased risk of exacerbations and even of asthma deaths.32 This is explained in part by reliance and escalating use of SABA and an associated delay in seeking medical care.33 Remote detection of excessive SABA use as a trigger to timely medical interventions is a potential application for novel digital platforms currently under development.
An alternative approach to reliever use, now recommended as the preferred option in the 2019 and 2020 GINA guide, is the use of an ICS/formoterol combination inhaler. The basis for this is that increasing symptoms in asthma usually reflects worsening airway inflammation, indicated, for example, by an increased level of exhaled nitric oxide.34 In experimental rhinovirus infection in patients with moderate asthma, symptoms of the common cold peak at Day 5, and asthma symptoms at Day 7.35 The latter are associated with large increases in cytokines, reflecting Type 2 inflammation: IL-4, IL-5, and IL-13. The prompt administration of the ICS-containing reliever (effectively an ‘anti-inflammatory reliever’) ensures that both symptoms and the cause of symptoms are addressed, as well as preventing severe exacerbations.36 This effect is evident in analyses of exacerbation risk in the days following reliever use: high symptom-prompted reliever use is an indicator of high exacerbation risk. This is considerably reduced if the reliever contains an ICS.37 The level of day-to-day asthma symptom control with this regimen is similar to that of the fixed maintenance dose plus SABA regimens.37 On the basis of four recent clinical trials on the use of as-needed ICS/formoterol without daily controller, this treatment is now recommended by GINA as the preferred approach in mild asthma. In the ‘real-life’ NOVEL START study, patients using an anti-inflammatory reliever required fewer courses of oral glucocorticoids, and had a lesser need of urgent medical care than both those taking SABA as-needed alone, and patients who received low-dose maintenance budesonide plus SABA as-needed.36 Prof Bateman concluded with the reminder that exacerbations occur in all severities of asthma and that maintenance and reliever treatment leverages normal patient behaviour when faced with symptoms. Use of an anti-inflammatory reliever titrated in this way provides a safer option, a ‘safety net’, preventing a large proportion of severe attacks across the range of asthma treatment steps.
Summary
Professor Eric Bateman
Many factors should affect the selection of inhaler devices. The patient’s mastery of the device and preference must be taken into account, but prescribers should consider selecting devices that have the lowest impact on the environment. By selecting devices with a high resistance, inspiratory flow is rarely a limiting factor because a low flow is sufficient for optimal results. There is an urgent need to formalise, and make mandatory, training in the use of inhaler devices for HCP. Proficiency of both HCP and patients is essential for successful therapy. Finally, maintenance and reliever therapy, which simplifies treatment by requiring a single inhaler for both maintenance and relief of symptoms, and leverages patients’ normal tendency to self-medicate when symptomatic, has been shown to improve ICS coverage, especially in patients who have faltering adherence.