Meeting Summary
In recent decades, there has been growing interest in the recognition and management of both bronchiectasis and nontuberculous mycobacteria (NTM) pulmonary disease. More specifically, interest in diagnosing NTM infection in patients with bronchiectasis has dramatically increased. Publication of the European Respiratory Society (ERS) guidelines and results from a number of large clinical trials have resulted in an exciting year for bronchiectasis research. Despite the increased knowledge and expanding options for disease management, a number of challenges persist. There remains a paucity of evidence to support management recommendations, which have not kept pace with the growing attention given to these diseases. To explore these limitations, Prof Chalmers summarised the reasoning behind the new guidelines.
The main objectives of these two presentations were to provide an expert overview of the challenges and achievements in the management of bronchiectasis and NTM pulmonary diseases, as well as predicting future trends. Dr van Ingen called for caution when managing these diseases because neither bronchiectasis nor NTM pulmonary disease can be described as single disease entities and, therefore, cannot be treated as such. The presence of NTM pulmonary disease is often a sign of multiple underlying conditions that must be addressed in tandem with culture conversion. Likewise, bronchiectasis pathogenesis is complex and failure of antibiotic therapy to offer consistent clinical benefit suggests infection is not central to pathogenesis in all patients, and a holistic approach is required. Finally, these interactive sessions uncovered and discussed various aspects and attitudes associated with disease management and highlighted how quality of care may be closely linked to clinical outcomes.
Current Knowledge Gaps in the Treatment of Bronchiectasis and Nontuberculous Mycobacteria Pulmonary Infection
Bronchiectasis is a serious, chronic, progressive pulmonary disease that is common in Europe. Most recent prevalence estimates are >400 cases per 100,000 men and >500 cases per 100,000 women, with a 40% increase in the past decade.1 Despite this, significant knowledge gaps prevail in key areas, including aetiology and pathogenesis. NTM infection is a considerable challenge for patients with bronchiectasis, with the overall prevalence reported as 9.3% in England, Wales, and Northern Ireland.2 Hence, there has been a rapid rise in research interest for the treatment and pathogenesis of bronchiectasis, and in the potential involvement of NTM pulmonary disease.
Bronchiectasis and NTM pulmonary disease often coexist and overlap, meaning disease evaluation can be challenging and pathogenesis pathways remain unclear. Combining antibiotic regimens is the current standard therapy for patients with bronchiectasis and NTM infection, but treatment has been predominantly guided by observational data, with just a few randomised controlled trials (RCT) providing evidence. In September 2017, the publication of new ERS guidelines for bronchiectasis was universally welcomed; however, the lack of evidence-based therapy options for patients is an ongoing challenge for healthcare professionals.
Bronchiectasis: The New, The Old, and The Ugly
Professor James D. Chalmers
In the past 12−24 months, bronchiectasis has garnered increasing interest, with the publication of data from the European Bronchiectasis Registry offering an insight into the natural history of bronchiectasis and a greater understanding of the complexities of its heterogeneity, which may provide potential applications in disease management. However, the evidence base has fallen short and, as yet, does not mirror the clinical importance of the disease. One major new development contributing to the growing knowledge of bronchiectasis is the publication of new ERS guidelines for the management of bronchiectasis in adults. The European bronchiectasis guideline was a multinational effort, uniting experts from many different disciplines. The guideline provides recommendations on the key aspects of bronchiectasis management across nine main areas: aetiology, exacerbations, eradication, long-term anti-inflammatories, long-term antibiotics, long-term mucoactives, long-term bronchodilators, thoracic surgery, and respiratory physiotherapy.3
Although aetiology may be viewed as a diagnostic effort, the decision was taken to include this topic in management recommendations to emphasise the importance of identifying treatable underlying causes of bronchiectasis. The first step in management is identifying the underlying cause of bronchiectasis and, when a treatable aetiology is identified, having robust guidelines to correctly treat the patient. One of the most controversial areas is management with long-term antibiotics, which has generated a large amount of debate. Years of cystic fibrosis (CF) research have proved the effectiveness of inhaled antibiotics for Pseudomonas aeruginosa infection, while data from three small but convincing studies published in the last 10 years have pointed towards the effectiveness of long-term macrolide use in bronchiectasis.4-6
Specific guidelines for patients with frequent exacerbations were included because, to date, the best evidence on prophylactic therapy supports exacerbation control. The guidelines emphasise that the first step is optimising airway clearance to reduce exacerbations, as many patients can control their disease with appropriate drainage of secretions from the lung. The next step, treatment of the infection, requires the exclusion of other causes of exacerbations, such as allergic bronchopulmonary aspergillosis or immunodeficiency, because allergic bronchopulmonary aspergillosis, for example, cannot be treated with an inhaled antibiotic. To counter this, a subclassification of bronchiectasis patients was introduced: those with or without P. aeruginosa infection. The guidelines recommend inhaled antibiotics for the treatment of patients with P. aeruginosa infection and oral antibiotics, predominantly macrolides, for the treatment of frequently exacerbating patients without P. aeruginosa infection. They also recommend avoiding macrolide monotherapy when there is a history of NTM because of the risk of inducing macrolide resistance.
Exacerbation history is a strong predictor of future outcome. One recent study assessed the stability of exacerbation frequency, clinical predictors, and outcomes of patients with frequently exacerbating disease.7 Patients who have ≥3 exacerbations per year were found to have a probability of >60% of having ≥3 exacerbations in the following year, and this pattern was predicted to extend into Year 3. The study showed that exacerbation frequency is a highly stable characteristic because without intervention, exacerbation frequency will be maintained. The greatest morbidity and mortality rates were also seen in the ≥3 exacerbations group, with a significant difference in 5-year survival rates in this group compared with the rest of the patients.7 Thus, these patients should be identified as a different subgroup or phenotype and targeted for additional treatment, such as optimising airway clearance, considering other causes of deterioration, and, when appropriate, adding long-term antimicrobial therapy. As with CF, there is evidence that patients with P. aeruginosa infection have worse clinical outcomes than those without7 and that they differ from other patients with bronchiectasis with regard to severity, presentation, and prognosis. Hospitalisation rates for patients with bronchiectasis and P. aeruginosa infection are often high because antibiotic resistance means patients must be treated for exacerbations with intravenous agents. Infections with Haemophilus influenzae have also been shown to indicate a poor prognosis when persistent and may require more aggressive intervention.7
No current clinical trial data demonstrate effective eradication of Pseudomonas infection in patients with bronchiectasis, but antibiotic-based eradication is the accepted standard of care for CF patients. On balance and based on clinical practice outcomes, it is believed that eradication should be attempted for patients with bronchiectasis and guidance on treatment regimens has been included in the ERS guidelines. Another challenging area is recommendations for bronchodilators and inhaled steroids. From European registry data, 60−70% of bronchiectasis patients are treated with either inhaled steroids, bronchodilators, or both, although no RCT currently exist showing evidence to support either approach.8 The ERS guideline recommends that it is reasonable to trial bronchodilators in patients with significant breathlessness, but inhaled corticosteroids are not recommended, except in patients with coexisting asthma or those with chronic obstructive pulmonary disease (COPD).
The vast majority of recommendations in the European guidelines are conditional and most of the strong recommendations are negative, e.g., avoidance of statins for bronchiectasis due to clear evidence of adverse effects with statin use in two RCT.3 Recombinant DNAase for the treatment of bronchiectasis is not recommended due to results of trials from the 1990s that show it may increase exacerbations, thus, emphasising the difference between bronchiectasis and CF.3 The only strong positive recommendation in the guidelines states that patients with impaired exercise capacity should participate in pulmonary rehabilitation and take regular exercise due to the beneficial impact on airway clearance, and that physiotherapy is a positive intervention.3
The overall message from the guidelines indicates just how weak the evidence base is for bronchiectasis. Historically, there has been some doubt as to whether bronchiectasis is actually a disease.9 In spite of these debates, it can be concluded that all bronchiectasis patients follow a broadly similar course and, excluding patients who require a specific antimicrobial intervention, like patients with NTM pulmonary disease, may be regarded as a valid group of patients. Indeed, these guidelines were the first to be titled for bronchiectasis, rather than non-CF bronchiectasis.
Some of the more negative trials in bronchiectasis have led to calls for the re-evaluation of the pathophysiology of the disease. In the 1980s, Dr Peter Cole proposed the idea of the ‘vicious cycle’, in which, because of epithelial dysfunction and mucus hypersecretion, patients develop impaired ciliary clearance, which causes neutrophilic inflammation, bacterial virulence factors, and lung dysfunction infection, leading to epithelial dysfunction.10 The natural extension of this is that, if the bacterial infection is cleared using antibiotics, the cycle is broken and other aspects of the disease should improve. Recently, this hypothesis has been tested by assessing whether intervening with antibiotics in patients with high bacterial loads and airway infection improves both rates of exacerbation and quality of life. The Phase III RESPIRE I and II trials11,12 evaluated the efficacy and safety of ciprofloxacin dry powder for inhalation in patients with bronchiectasis 14 days on and 14 days off or 28 days on and 28 days off over 1 year. The results were conflicting, with RESPIRE I reporting a reduction in time to first exacerbation in the 14-day arm and RESPIRE II showing a non-significant trend to a reduced exacerbation rate in the 28-day arm. In September 2018, an alternative concept for bronchiectasis was published suggesting a more complex model for pathogenesis. The model has been termed the ‘vicious vortex’: when one pathway is blocked by eliminating bacterial infection the lung destruction continues to affect ciliary function and inflammation persists.13 Therefore, all aspects may be interdependent, so targeting one aspect in isolation, such as bacterial infection, is likely to only have a modest impact on clinical outcomes.
In light of these updates, where does the solution lie for the effective management of patients with bronchiectasis? Antibiotic therapy is still very important, although one form of intervention is often insufficient. Therefore, the concept of targeting the ‘treatable traits’ has become more relevant than ever to effectively manage these patients. Antibiotic treatment to combat bacterial infection is still a valid management strategy, but treating the underlying aetiology may be equally as important. Patients with bronchiectasis now require a multimodal treatment approach. Solving the vicious vortex lies in addressing treatable pulmonary, aetiological, extrapulmonary, and environmental traits, whenever possible.
Nontuberculous Mycobacteria: How to Treat Difficult Infections
Doctor Jakko van Ingen
“We now leave the safe haven of evidence- based medicine and slowly go down into the abyss when speaking of NTM disease,” began Dr van Ingen, referring to the challenges in treating difficult NTM infections. NTM pulmonary disease requires a multidisciplinary approach because, like bronchiectasis, it is not a single disease entity. Instead, it can be described as at least two very distinct entities: fibrocavitary disease that tends to affect those with a history of pulmonary diseases with underlying COPD and silicosis, and nodular bronchiectatic disease that commonly affects patients who do not have remarkable pulmonary history, except perhaps for bronchiectasis (Figure 1). Currently, both entities are treated in almost the same way, and this requires further consideration.
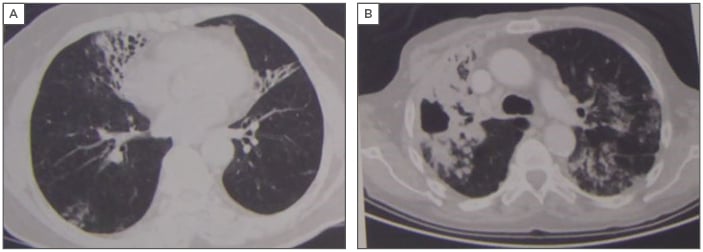
Figure 1: The two separate disease entities of nontuberculous mycobacteria pulmonary infection: nodular bronchiectatic (A) and fibrocavitary disease (B).
Dr van Ingen introduced a recent case study of a 59-year-old male with Mycobacterium avium-intracellulare pulmonary disease; the patient was an active smoker (35 pack-year history), presented with COPD, and had a Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of II. The patient presented with fatigue, cough, sputum, and weight loss of 9 kg, with 3X sputum acid-fast bacilli+++ and 3X sputum culture indicating M. intracellulare infection. In treating NTM pulmonary disease, the practitioner must first assess the patient as a pulmonologist by focussing on environmental and underlying conditions, including smoking cessation. It is clear from studies of tuberculosis that active smoking delays culture conversion and leads to poor treatment outcomes,14 so the same would apply for M. intracellulare infections. As a pulmonologist, the initial step in the management of NTM pulmonary disease can be achieved by asking pertinent questions, such as ‘why has the patient developed NTM pulmonary disease?’, ‘what is the burden of treatment?’, and ‘does such a treatment burden outweigh the current burden of disease?’. The potential challenges regarding regimen compliance must be addressed for each patient, along with any predictable drug–drug interactions. Any potential role for surgical resection of the worst affected areas must be considered, especially in light of high rates of recurrence and relapse of NTM pulmonary disease.
Current treatment guidelines for NTM pulmonary disease include recommendations published by the American Thoracic Society (ATS)15 and the British Thoracic Society (BTS).16 According to these guidelines, Mycobacterium avium-complex (MAC) pulmonary disease (MAC-PD) should be treated with rifampin and ethambutol plus a macrolide (RIF-EMB-macrolide) with or without 3 months of intravenous amikacin. For Mycobacterium abscessus, an intravenous and oral continuation phase are merited, consisting of amikacin, imipenem, tigecycline, and azithromycin, followed by azithromycin, clofazimine, and 1 or 2 other oral or inhaled agents, guided but not dictated by in vitro susceptibility testing.17 Retrospective analyses of case series have shown that prolonged culture conversion can be achieved using the recommended RIF-EMB-macrolide-based regimen in >70% of MAC-PD patients, but that macrolide resistance and fibrocavitary disease are risk factors for treatment failure and conversion rates drop to 40–50% in these patient categories.17 It is important to note that macrolide monotherapy should never be administered due to potential development of resistance. Macrolide-fluoroquinolone combinations have also been shown to promote macrolide resistance.18
It is interesting to note whether these guideline recommendations are reflected in real-world treatment methods. One survey of five European countries (France, Germany, Italy, Spain, and the UK) and Japan measured guideline adherence for the treatment of NTM.19 Altogether, 619 physicians (Europe: 446; Japan: 173) participated, covering 1,429 cases (Europe: 1,012; Japan: 417). The key finding from the survey indicated that few MAC patients receive RIF-EMB-macrolide for >6 months. In fact, on average in Europe, only 9% of MAC patients received the guideline recommended regimen, compared to 42% in Japan,19 which indicates enormous room for improvement. However, it is unknown whether such low levels recorded were due to intolerance rather than a knowledge gap.
Dr van Ingen returned to the previous case study and indicated that this patient was intolerant to rifampicin and discussed alternative treatment regimens. One alternative regimen option is treating with rifabutin, but intolerance may be difficult to anticipate because the drug has very specific toxicities and many interaction challenges. Clofazimine experience in MAC-PD is mainly limited to two combination strategies, each with benefits and weaknesses. Clofazimine-minocycline-clarithromycin combinations were assessed in 22 patients, producing a 64% prolonged culture conversion.20 The second option, clofazimine-ethambutol-macrolide, was assessed in 90 patients and, while a 100% culture conversion was achieved, there was a 37% relapse rate.21 Another option to be considered is switching to bedaquiline-ethambutol-azithromycin, which has shown moderate in vitro activity. For example, when assessed in six MAC-PD patients and given with rifampicin, symptomatic improvement was observed.22 However, microbiological failure also occurred in four patients due to bedaquiline resistance.22 Therefore, the efficacy of bedaquiline remains very uncertain.
More recently, clinical trial data were published on the use and efficacy of amikacin liposome inhalation suspension (ALIS) for refractory MAC-PD.23 In 336 patients with refractory MAC-PD, 224 received guidance-based therapy (GBT) plus ALIS, while 112 were treated with GBT alone. Culture conversion occurred in 29% receiving ALIS+GBT versus 9% in those receiving GBT alone (p<0.001).23 While this is an exciting result, it should be noted that in the other 70% of patients, microbiological outcomes were not improved, indicating the extent of the challenge ahead. Such outcomes have not been mirrored when treating M. abscessus infection and it may be advisable to consult expert centres for guidance when M. abscessus infection presents. This is of particular note in the continuation phase of treatment, when the combination choices noted previously have demonstrated in vitro activity against M. abscessus. In one study in the USA, 33 of 69 M. abscessus patients (48%) treated with individualised treatment regimens based on drug susceptibility results and patient tolerance following ATS/Infectious Diseases Society of America (IDSA) recommendations achieved culture conversion.24
Tigecycline, a glycylcycline antibiotic with broad-spectrum antimicrobial activity, now features in guidelines and may slightly improve outcomes. In a study of tigecycline for compassionate use, 16 out of 26 pulmonary cases improved.25 It should be noted that bacterial subspecies influence clinical outcomes of tigecycline treatment. Across the retrospective case series, 25% of patients treated for pulmonary disease caused by M. abscessus subsp. abscessus achieved culture conversion, while for disease caused by the macrolide-susceptible M. abscessus subsp. massiliense, culture conversion was attained in 70−80% of affected patients.25 Dr van Ingen’s case study illustrates the challenges regimen intolerance may pose to treatment and the need to address regimen strengths and weaknesses in each case.
Conclusion
In summary, prevalence estimates for bronchiectasis have increased by 40% in the last 10 years, yet the evidence base supporting diagnosis and treatment has not kept up with the clinical importance of the disease. The recent publication of ERS guidelines for bronchiectasis has offered important guidance on management and, with careful trial design, it is hoped further clinical results will become available to add to the evidence for treatment recommendations. In relation to the model of bronchiectasis pathogenesis, all aspects may be interdependent and, as such, targeting only the bacterial infection is likely to have a modest impact on clinical outcomes.
When treating NTM pulmonary disease, the recent results showing effectiveness of ALIS treatment for refractory MAC-PD are inspiring and multiple trials with clofazimine are ongoing; however, few new developments have occurred for the treatment of M. abscessus infection. Therefore, it is always advisable to treat NTM pulmonary disease according to current ATS/IDSA guidelines. In the treatment of NTM pulmonary disease, addressing treatable pulmonary, aetiological, extrapulmonary, and environmental traits, whenever possible, along with infection should achieve more favourable outcomes.








