Meeting Summary
Patient outcomes must take precedence when considering environmental legislation related to the availability of inhaler devices, which are essential for the care of patients with respiratory diseases.
This article reviews presentations and abstracts from the European Respiratory Society (ERS) International Congress 2023, held in Milan, Italy, in September 2023. The sessions focused on healthcare inequality and patient outcomes, highlighting the need for stakeholders to make patient-centric decisions in order to ensure access to essential inhaled medicines are prioritised. This is especially important during a period when there is an increasing need to reduce the carbon footprint associated with respiratory care.
During a satellite symposium, co-chairs John Hurst, Professor of Respiratory Medicine at University College London (UCL), UK, and Helen Reddel, Research Leader at the Woolcock Institute of Medical Research, Sydney, Australia, emphasised the necessity of addressing environmentally sustainable respiratory care while prioritising patient outcomes.
Christine Jenkins, Clinical Professor of Respiratory Medicine at the University of New South Wales (UNSW), Sydney, Australia, discussed the association between health inequity and uncontrolled chronic obstructive pulmonary disease (COPD) and asthma, and how that relates to the carbon footprint of treatment. Alberto Papi, Full Professor of Respiratory Medicine at the University of Ferrara, Italy, examined how implementing evidence-based guidelines can improve patient outcomes and reduce the carbon footprint of respiratory care, and the progress being made in the transition to near-zero propellants in pressurised metered-dose inhaler (pMDI) devices. Omar Usmani, Professor of Respiratory Medicine at the National Heart and Lung Institute (NHLI), Imperial College London, UK, stressed that pMDIs contain essential medicines, and inhaler regimens should not be considered readily interchangeable. He urged the respiratory community to ensure that their voice is heard in decisions where it relates to the environment regarding COPD and asthma care.
The symposium emphasised the opportunities to reduce the environmental impact of respiratory care whilst prioritising patient outcomes. By supporting the transition to climate-friendly propellants in pMDI devices, and implementing guidelines to improve patient outcomes, the overall carbon footprint of respiratory care can be reduced. However, this must be done without limiting access to essential medicines, or increasing adverse health outcomes. The symposium identified pathways towards achieving patient-centric, sustainable respiratory care by improving outcomes, harnessing innovation, and promoting multi-stakeholder collaboration.
Introduction
John Hurst
Hurst set the scene by highlighting that policymakers face significant challenges in addressing the post-COVID-19 priorities of resolving health inequity, building system resilience, and reducing greenhouse gas (GHG) emissions.1 Improvements in respiratory care have stalled in recent years.2 He noted that this slow-down has not occurred universally amongst all patients living with respiratory disease; rather, a disproportionate burden of mortality and morbidity due to respiratory disease occurs in people from socioeconomically deprived areas.3,4 The problem is getting worse, as an ageing population is contributing to rising disease prevalence.5 In parallel, there is climate change. Hurst stated that if the global healthcare sector were a country, it would be the fifth-largest emitter on the planet.1
Respiratory disease is a key driver of the healthcare sector’s GHG emissions. Nonetheless, inhaled medicines are essential for patients living with COPD and asthma.6 Hurst emphasised that pMDIs are the most commonly used inhaler device,7 and the sole available option for children and for many of those living with COPD. However, pMDIs have faced scrutiny, as the propellants they contain have a high global warming potential (GWP).8,9 As a result, Hurst highlighted calls, including legislative proposals, seeking to reduce their use. However, he emphasised that lower-emission solutions should not compromise the quality of patient care,10 and unintended consequences of jeopardising improvements in outcomes.11
This congress review highlights the factors driving carbon emissions in respiratory treatment, and examines the links between socioeconomic inequalities, poor disease control, and environmental impact. The review also shines a spotlight on how the respiratory community must use its voice to ensure that patients remain central to any decisions that may impact the availability of treatments.
Facing the Challenges for Healthcare in the 21st Century: Health Equity, System Resilience, and the Environment
Christine Jenkins
There is a high burden of uncontrolled disease in those living with COPD and asthma around the world. Jenkins highlighted that in 2019, COPD was the third most common cause of death globally, accounting for approximately 3 million deaths.12 In the UK, COPD is the second most common cause of emergency admissions.13 For those with asthma, approximately 40% of patients across countries, regardless of their severity of asthma, are potentially over-reliant on short-acting β-agonist (SABA) relievers, which is associated with severe exacerbation risk, independent of inhaled corticosteroid maintenance use.14 Additionally, a short course of systemic corticosteroids every 1–2 years is associated with an increased risk of adverse health conditions, such as osteoporosis, cataracts, and diabetes.15
A high proportion of the burden of COPD and asthma lies in low- and middle-income countries (LMIC), where access to good care is limited,16-18 while in high-income countries (HIC), socioeconomic disparities also contribute to poor outcomes.3,4 Jenkins noted that for asthma, 90% of the burden of disease is borne by people living in LMICs,16 and steroid inhalers are only available in one-third of public primary healthcare facilities in LMICs.18 In many low-income countries, the availability of asthma medicines is low, with only 30.1% in the public healthcare system and 43.1% in private sectors receiving medications.17
Jenkins pointed out that social deprivation is associated with worse outcomes from respiratory conditions, both in terms of morbidity and mortality. In LMICs, COPD mortality is most prevalent in regions with limited resources, particularly in countries with a yearly gross national income of less than 20,000 USD per capita.19 However, HICs are not immune, with social deprivation having an impact on outcomes. COPD data from the USA show that the most deprived areas exhibit the highest likelihood of exacerbations, and an increased incidence of severe exacerbations.3 In one study from Germany, more than one-third of patients with COPD did not receive maintenance therapy following either one severe or multiple exacerbations.20 For patients with asthma, data from the UK show an elevated likelihood of emergency admissions and longer length of hospital stay with increasing deprivation.4
Jenkins highlighted how the prevalence of respiratory disease increases in both males and females in later life, especially from the age of 60 years.5 This is relevant since, according to the World Health Organization (WHO), the global population aged 60 years and older is expected to double, from 1.0 billion in 2020 to 2.1 billion by 2050.21 An even greater burden of chronic respiratory disease is, therefore, anticipated in both LMICs and HICs. This emphasised all the more the urgency to address the already considerably high prevalence of respiratory disease globally.
The Carbon Footprint Across Healthcare
All aspects of healthcare possess a carbon footprint, with one-quarter of these GHG emissions related directly to the delivery of care.9 Therefore, the choices made in patient care have a direct impact on healthcare emissions.9 In the UK, the GHG emissions associated with healthcare activities, including travel, have been defined.22 The GHG emission associated with a general practitioner visit amounts to 1.1 kg of CO2 equivalent (CO2e).22 This figure rises progressively with the increasing intensity of treatment; for instance, if patients require emergency department care (14 kg CO2e), or if they require a hospital stay in a low- or high-intensity bed ward (38 and 90 kg CO2e, respectively).22 Jenkins emphasised that healthcare-related GHG emissions would, therefore, be amplified for those with poorly-controlled disease who require urgent or emergency care.
Jenkins presented data from around the world, illustrating the high proportion of SABA reliever medication use in many countries, which the authors had highlighted is indicative of sub-optimal management of respiratory disease being commonplace. This has a consequence in terms of GHG emissions. The SABA CARBON (Healthcare-based Carbon Cost of Treatment) Europe and Canada observational study by Janson et al.8 reviewed inhaler sales data obtained from the IQVIA (Durham, North Carolina, USA) quarterly MIDAS® database as a surrogate for inhaler use (MDIs and dry powder inhalers), for SABA and controller medications (inhaled corticosteroid [ICS]-containing drugs, long-acting β2-agonists [LABA], long-acting muscarinic antagonists [LAMA], and LAMA/LABA combinations). Across the 21 countries included, SABA use was common.8 The study identified that SABAs accounted for two-thirds of the total GHG emissions, contributing 2 million tonnes of CO2e, while the use of controller inhalers resulted in approximately 1 million tonnes of CO2e (Figure 1).8 In six countries (Canada, Germany, Italy, Poland, Sweden, and the UK), analyses showed that between 69–94% of SABA prescribing/dispensing in asthma were for patients who required three or more reliever inhalers in a 12-month period, and who were potentially uncontrolled.8 This had an environmental consequence of between 78–864 tonnes of CO2e per 10,000 persons per year.8 Any additional healthcare demand arising from the consequences of uncontrolled asthma would be associated with a carbon footprint.8 This is true globally, with data from the SABA CARBON International study, conducted in Africa, Asia Pacific, Latin America, and the Middle East, showing the same pattern.23 For example, in Australia, SABAs represent 83% of use and 87% of the GHG emissions of inhalers.23 Jenkins highlighted that if these patients’ symptoms could be better controlled, then this would reduce the requirement for SABA, with the attendant environmental benefits.
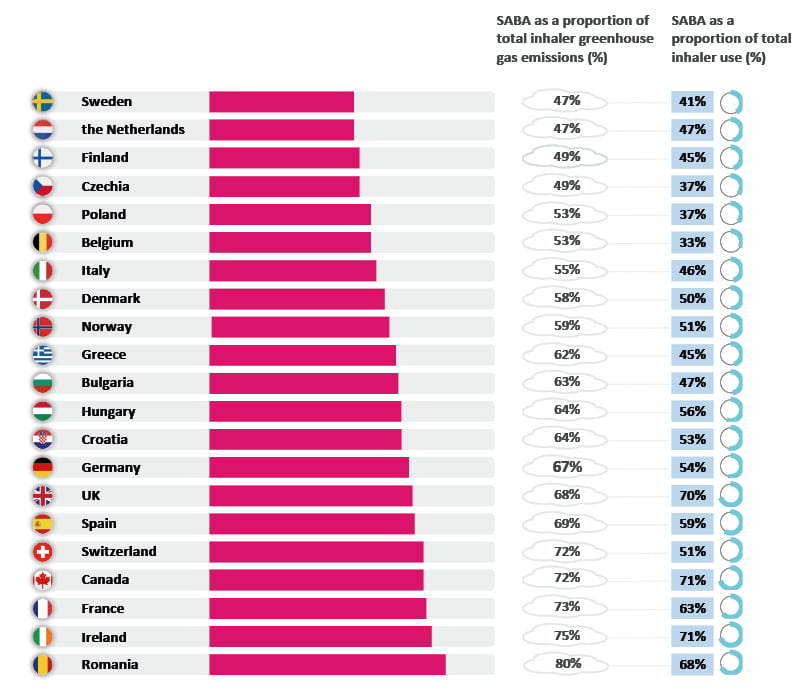
Figure 1: Inhaler sales data, as a surrogate for inhaler use, for short-acting β-agonist and controller medications, across all respiratory uses, from the IQVIA (Durham, North Carolina, USA) quarterly MIDAS® database Q3 2019 (September 2018–September 2019).8
Adapted from Janson et al.8
Q: Quarter; SABA: short-acting β-agonist.
The SABINA CARBON study looked at all aspects of asthma healthcare, scaled to the UK asthma population.24 This included healthcare resource utilisation (HCRU) and prescribing. It found that patients who were uncontrolled in their asthma disease, as defined by recent exacerbation history or over-reliance on reliever (SABA) prescribing, had a three-fold higher carbon footprint than those who were well-controlled.24 Treating the consequences of poor control accounted for 45% of the total carbon footprint, 303,874 tonnes of CO2e per year, which is equivalent to emissions from more than 124,000 houses in the UK.25
A further analysis from SABINA CARBON investigating socioeconomic disparities and the carbon footprint of asthma care was presented as a poster at ERS, by Ekaterina Maslova, Director of Epidemiology at AstraZeneca, Cambridge, UK. The findings demonstrated that the carbon footprint of asthma care in the UK increased with higher socioeconomic deprivation.26 Specifically, patients with a higher Index of Multiple Deprivation (IMD) score (where scores range from least to most deprived, and higher scores are indicative of higher deprivation) exhibited GHG emissions that were approximately two-fold higher compared with those with a lower IMD score.26 This suggests that there is an environmental consequence that comes from socioeconomic disparities, in addition to the previously reported observations of higher carbon footprint in patients who are uncontrolled. The study also showed that overall GHG emissions per capita related to asthma care were driven by the prescription of SABA, underscoring the need to proactively optimise maintenance therapy in accordance with current evidence-based recommendations, to reduce reliance on reliever inhalers.26 By effectively implementing treatment recommendations that prefer the use of anti-inflammatory relievers to improve control of symptoms and prevent exacerbations, healthcare systems could achieve improved and sustained clinical outcomes, subsequently reducing the overall carbon footprint of asthma care.26 The authors concluded that targeting quality improvement programmes to areas of higher deprivation, with the goal of removing disparities in care and improving patient outcomes, could also be an important route for greater savings in the CO2e emissions associated with asthma care.
Jenkins also illustrated the relationships between COPD exacerbations, HCRU, and carbon footprint. A history of COPD exacerbation increases the likelihood of a future exacerbation. For those with a severe exacerbation, this risk is higher still, so it is crucial that patients who are hospitalised are discharged on the appropriate medication to reduce the risk of recurrence. However, a study in Germany showed this continuity in care was not experienced by over one-third of patients.20 A total of 37% of patients did not receive appropriate guideline-directed controller medications (LAMA, LABA, or ICS), following either one severe or multiple (regardless of severity), COPD exacerbations.20 She emphasised that people are being “grossly undertreated for a very serious disease.”
Data from the SHERLOCK study showed that even one moderate exacerbation increases the risk for subsequent exacerbations, compared with having no recent exacerbation.27 For patients with COPD who experience a severe,
or multiple exacerbations, the risk was higher still.27 A subsequent analysis of the GHG emissions associated with the HCRU and SABA prescribing from SHERLOCK CARBON showed that these, too, increased.28 Compared with patients with no history of exacerbations in the baseline year, a history of ≥2 moderate and/or severe exacerbations increased GHG emissions by 51%, 51%, and 44% at 12, 24, and 36 months, respectively.28 Jenkins noted that this indicates the potential that prompter intervention following COPD exacerbations may have, not just in reducing future exacerbations, but also by reducing the GHG emissions associated with care.
In conclusion, Jenkins illustrated the links between health inequalities, uncontrolled respiratory disease, and carbon footprint. She identified that all elements of healthcare contribute to the carbon footprint, and that SABA relievers account for the majority of GHG emissions from inhalers. Attention should be directed towards evidence-based approaches and guideline implementation, such as controller medications, to prioritise disease management and improve patient outcomes, whilst supporting a reduction in the carbon footprint of care.8
Paving the Way for Environmentally Sustainable Respiratory Care
Alberto Papi
Papi highlighted the opportunities for reducing carbon emissions of respiratory conditions. Two main areas were discussed: implementing recommendations and evidence-based approaches to improve outcomes in patients with COPD and asthma,29-32 and transitioning to more climate-friendly pMDI inhaler medicines.
Implementing Evidence-Based Approaches and Recommendations
There are clear evidence-based treatment recommendations for asthma and COPD, as published by the Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD).33,34 These recommendations aim for personalised treatment by assessing patient status and adjusting therapy.33,34 Papi identified that implementation of these recommendations is important to improve outcomes and, as a consequence, would be expected to reduce the CO2 emissions associated with care.
For instance, acute exacerbations of COPD are associated with frequent readmissions.29 An approach to reduce readmissions of patients with COPD discharged from hospital was implemented in Canada.29 Patients were provided with a simple seven-item tool (including, for example, inhaler technique, treatment optimisation, and smoking cessation) to facilitate care continuity.29 Patients receiving the transition bundle were 83% less likely to be readmitted within 7 days (relative risk: 0.17; 95% confidence interval: 0.07–0.35; p<0.001), and 26% less likely to be readmitted within 30 days of discharge (relative risk: 0.74; 95% confidence interval: 0.60–0.91; p=0.06) compared with the usual care cohort.29
PRIMUS, a retrospective observational study utilising USA healthcare insurance claims data (N=24,770), demonstrated that the prompt initiation (≤30 days post-index) of GOLD-recommended triple therapy (comprising ICS, LABA, and LAMA) following one severe or ≥two moderate COPD exacerbations, was associated with a reduced risk of future COPD exacerbations (occurrence and number of exacerbations), decreased morbidity, and a reduction in COPD-related costs during the 12-month follow-up period compared to delayed (31–180 days) or very delayed (181–365 days) triple therapy.30 The study indicated that implementation of management guidelines is warranted to prevent future exacerbations, and to reduce the economic burden among patients with COPD.30 Papi said that implementing guidelines minimises the impact of exacerbations, future demand on healthcare, and would impact the associated carbon emissions, too.
Papi believed that implementing GOLD-recommended treatment has the potential to impact the resilience of healthcare systems and enhance patient outcomes. The PROMETHEUS study, a simulation-based projection utilising a modelled USA COPD population with 1,000 simulations of patient progression, was designed to estimate the long-term exacerbation and mortality benefits of implementing fixed triple therapy.31 The study predicted that implementation would reduce COPD exacerbation-associated hospitalisations by 2 million, extending the average life expectancy of patients by 2.2 years, and contributing to an additional 3 million years of life to the USA population over the span of 10 years.31 In addition to the obvious benefit to patients and demand on emergency care, the improvements would also be expected to have an environmental benefit.
Regarding asthma care, the ongoing SENTINEL programme aims to reduce SABA overuse through the supported implementation of a local adult asthma guideline, which advocates a SABA-free, maintenance and reliever therapy-preferred strategy for patients with uncontrolled asthma.32 The study was designed to improve asthma outcomes, decrease SABA overuse, and reduce the environmental impact of asthma and its treatment. The study is being conducted in a network of practices in one of the most deprived areas in England, which was in the 90th decile of SABA prescribing.32 Patients are prioritised for asthma review based on risk, which includes the level of SABA prescribing (n=2,571).32 After 12 months, the patient-tailored approach was associated with improved asthma outcomes; a reduction of high SABA overuse (≥6 SABA prescriptions in 12 months) from approximately 30% to 13%; and, for those who underwent treatment optimisation, there was a 30% reduction in the proportion of patients experiencing one or more exacerbations.32 Importantly, the authors believe this is the first prospectively designed study to investigate both a change in outcomes and GHG emissions resulting from quality improvement.32 The full analysis of the GHG emissions associated with care is pending; however, interim data are available for the impact of the programme on SABA prescribing.35 In the first 12 months following the inclusion of the last site to participate in the quality improvement programme, there were an estimated 44,275 fewer SABA relievers issued for the treatment of asthma, which amounts to a saving of 1,240 metric tonnes of CO2e, the equivalent to 1,550 transatlantic flights from Leeds, UK, to New York, USA.35 A fuller picture of the carbon reduction associated with guideline-recommended treatment will be revealed in future analyses, which will account for the GHG emissions linked with the fewer asthma exacerbations.
Transitioning to Next-Generation Propellants That Are More Climate-Friendly
Papi then moved on to describe the ongoing work to reduce the carbon footprint associated with pMDIs. All pMDIs contain a propellant, and those currently approved have a high GWP, meaning they are greenhouse gases. Hydrofluoroalkane (HFA)-227ea and HFA-134a, have a 100-year GWP of 3,140 and 1,360, respectively.36 However, next-generation propellants are in development, which have either low or near-zero GWP, and with a reduction of up to 99.9% GWP compared with propellants in use currently.37 A reason for the reduced GWP was shown to be the time the gaseous propellant is present in the atmosphere before degrading. For the older propellants, HFA-227ea and HFA-134a, this is 36 and 14 years, respectively, compared with 1.6 years for HFC-152a, and 19 days for HFO-1234ze(E).36 For pMDIs using HFO1234ze(E), the carbon footprint of the device would be similar to a dry-powder inhaler (DPI). From 2025 onwards, pMDIs containing these next-generation propellants will become available.38
Ensuring Access to Essential Medicines: Why Partnership with Governments and Authorities is the Only Way to Achieve Patient-Centric Environmentally Sustainable Respiratory Care
Omar Usmani
The carbon footprint associated with pMDIs has prompted some individuals and groups to call for limits to their availability.39 However, although the impact of pMDIs on global GHG emissions is important,38 they are a relatively small generator of anthropogenic CO2, contributing <0.04% (Figure 2).40 Usmani stressed the need for a pragmatic approach that puts patients first, and ensures that they have uninterrupted access to essential inhaler medicines. In that regard, making the transition to environmentally-friendly propellants in pMDI devices is important to securing the continuity of patient care.41
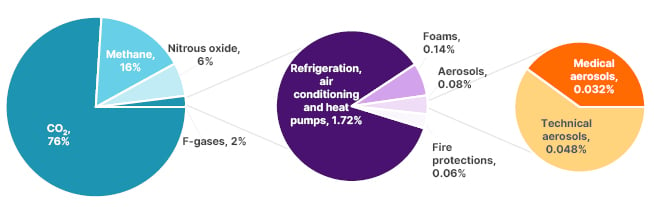
Figure 2: The share of medical aerosols in the total global greenhouse gas emissions (data from United Nations Environment Programme, The Montreal Protocol, 2016).40
Adapted from Emeryk et al.40
Switching Inhaler Regimens is a Complex Issue
Usmani said that the impact of inhalers on the environment is more complex than just considering carbon emissions. There are multiple ways that inhaler devices impact the environment that go beyond their GHG emissions alone, and where, across a number of factors, a DPI has a greater impact. Jeswani and Azapagic42 showed that the transition of pMDIs to a next-generation propellant would have a less harmful impact on the environment than switching to dry powder inhalers when considering these factors together. The alternative of switching from pMDIs to DPI to would lead to a greater environmental impact on factors, such as photochemical oxidants formation, fossil depletion, and marine ecotoxicity, which contribute to terrestrial pollution and marine eutrophication.42 These effects occur throughout the lifecycle of inhalers, from the manufacturing process to their disposal.42
Usmani noted that the delivery of drugs by inhalation is an integral component in the management of patients with COPD and asthma, and emphasised the importance of the “voice of the patient.”
The correct use of inhalers, and adherence to prescribed regimens, are key aspects in achieving better clinical control and enhanced quality of life.43 Personalising treatments, with the tailored selection of devices, enhances patient satisfaction,44 treatment adherence, and clinical outcomes,45 where the choice of an inhaler device is as important as the choice of medications it contains.46 Patient empowerment is key to optimal management and disease control, whereby patients who are consulted and satisfied with their device are more likely to use it, and benefit from the therapy.47
Usmani noted that pMDIs play a critical role in respiratory medicine, representing the majority of inhaler usage. Certain groups of patients have no alternative to pMDIs. Children, for example, are often solely dependent on pMDIs as indicated treatment.48 In the emergency setting, pMDIs are prioritised for use by GOLD and GINA.33,34 Inhaled medicines delivered by pMDIs are included on the WHO Essential Medicines List.6 This is particularly important for LMICs. A study presented at the American Thoracic Society (ATS) International Conference, in May 2023, assessed pMDI use as a proportion of all inhalers, in 53 countries spanning six geographical regions.49 It showed that pMDI use accounted for 78% of all inhaler use, and represented the majority of inhaler use in 49 countries.49 In 17 of 20 European countries studied, pMDIs accounted for 75.6% of all inhaler use, rising to more than 90.0% in countries including Colombia and Peru.49
Usmani highlighted that switching inhaler regimens, advocated by some, is a complex issue, with variable clinical and patient-related consequences.50 He pointed out that inhaler regimen switches can be detrimental to the patient–healthcare professional relationship, especially when the switches are non-consented.11 Furthermore, he made the point that patients who experience a severe COPD exacerbation have a similar mortality prognosis to those with heart failure, acute myocardial infarction, or bladder cancer, who would not be denied effective treatments on the basis of carbon footprint.
Advocating for Patient Wellbeing During Policy Development
Advocacy by the respiratory community has an important role in evidence-based policy development. Incentivisation to switch inhalers was stopped in the UK in April 2023, which Usmani said may have been partly due to the respiratory community explaining to the government and policymakers that switching may be inappropriate.51 He added that the greenest inhaler is the one that the patient can, and will, use correctly.52
An expert consensus poster was presented at ERS by Tonya Winders, President and CEO of Global Allergy and Airways Patient Platform (GAAPP), Vienna, Virginia, USA. The aim was to develop global consensus quality statements on how and when to implement an appropriate inhaler regimen switch to inform clinical practice and health system policy around regimen switching.53 The consensus highlighted that the most important factors that should inform an inhaler regimen switch should be clinical and patient-led factors, such as inadequate disease control, non-adherence to regimens, and patient preferences, as well as proper implementation procedures, and inhaler technique.53 The consensus view was that a median of 35 minutes per patient was required for an effective inhaler switch consultation.53 This encompasses tasks such as identifying the need to switch, conducting assessments, providing training, documenting the switch, planning monitoring reviews, and allocating review time.53 The consensus emphasised that switches should be only initiated and implemented by qualified healthcare professionals.53
Usmani asserted that advocacy by the respiratory community has an important role in supporting evidence-based policy development. He pointed to the 30 individuals and groups who provided responses to the public consultation to the draft F-Gas Regulation legislation.51 This had been pivotal, he believed, in shaping the public positions of the European bodies, and the recognition of the importance of safeguarding access to essential medicines during the transition to low, or near-zero, GWP propellants. Usmani urged legislators to safeguard respiratory patients from the unintended consequences of any environmentally-led restrictions on essential medicines.
Usmani then highlighted a proposal submitted by five European Union (EU) countries to the European Chemicals Agency (EHCA), calling for restrictions on the manufacture and use of per- and polyfluoroalkyl substances (PFAS) from 2026.54 This proposal aims to minimise the release of molecules with long environmental persistence to protect against potential negative effects on the environment and human health in the future.55 However, PFAS molecules are used across many medicines’ lifespans, and play a role in the research, development, and manufacturing of lifesaving medicines.56,57 As such, they are already tightly controlled by health authorities.58 In particular, there is a risk to HFO-1234ze(E), which has been classified as a PFAS based on chemical structure, despite it possessing none of the properties that are the reason for the ban, i.e., it is non-persistent, non-bioaccumulative, and non-toxic.59 Usmani emphasised the importance of communicating to policymakers that transitioning to climate-friendly propellants is crucial for both patient care, and for addressing climate change.36,37
In conclusion, respiratory clinicians must remain vigilant to the unintended consequences of well-meaning environmental legislation, and should advocate for patients with local authorities, governments, policymakers, and stakeholders.
Summary and Closing
Helen Reddel
Selecting inhalers, and considering their impact on the environment, is intertwined with patient wellbeing, clinical outcomes, and global sustainability. While it is important to consider the environmental impact of pMDI devices, it is essential to prioritise personalised treatment based on an individual’s need.10
Reddel highlighted the importance of access to inhaled asthma medications, especially those in LMICs, who already experience limited access to these therapies.10 Shared decision-making between healthcare professionals and patients should focus on control of symptoms and reducing the risk of poor outcomes. Better control of disease impacts reliever use and HCRU, both of which have a carbon consequence.10
Reddel emphasised that patients in LMICs, underserved areas, and the elderly are the most affected by respiratory disease and climate change. Therefore, urgent action is required to improve individual patient management, which can influence the burden of disease experienced by patients, and their likelihood of requiring emergency healthcare.
New propellants for inhaler devices are currently under development. However, it is crucial that legislation does not deter innovation, and that there is collaboration among stakeholders to achieve sustainable respiratory care without compromising patient access or health outcomes. Reddel urged attendees to provide a submission to the PFAS public consultation, and to continue advocating for, and with, patients to maintain inhaler options for those with asthma, COPD, and other respiratory conditions. This will not only improve the health of patients, but also of the planet. This underscores the significance of ensuring the respiratory community’s voice is heard, preventing the unintended consequences that may arise from otherwise well-meaning and important legislation.







