Meeting Summary
This symposium took place during the 29th European Respiratory Society (ERS) Congress. Prof Rabe opened by describing the global burden of asthma, followed by Prof Humbert highlighting the importance of impaired lung function, the need for ongoing monitoring, and the clinical relevance of improvements. Type 2 inflammation promotes lung-function deterioration by driving structural and functional changes. Quality of life and daily activities are severely impacted by impaired lung function, which has close links with severe asthma exacerbations. Prof Rabe then explained Type 2 inflammation and its relationship with asthma pathology. He also described how IL-4/13 and IL-5 are key cytokines in the Type 2 inflammatory pathway, while IL-4/13 are central drivers of asthma pathophysiology associated with airway obstruction and leading to impaired lung function. Elevated fractional exhaled nitric oxide (FeNO), eosinophils (EOS), and serum IgE are Type 2 biomarkers and are driven by the activity of IL-4/13 and IL-5. Prof Wechsler emphasised the importance of identifying the type of asthma a patient has: eosinophilic, allergic asthma, Type 2 mediated, or
non-Type 2. He then provided an overview of biologics and their impact on lung function, exacerbations, and patient reported outcomes. Global Initiative for Asthma (GINA) 2019 guidelines recommend considering add-on of Type 2-targeted biologics at Step 5 and stress the need to
eliminate the use of maintenance oral corticosteroid (OCS) therapy. Prof Wechsler closed with research that showed dupilumab use has demonstrated improved lung function and reductions in exacerbations despite reduction of OCS use.
Welcome and Introduction
Professor Klaus F. Rabe
Asthma imparts a global burden with approximately 339.4 million people affected worldwide in 2016,1,2 a 17.2% increase from 2006.2 A large proportion of these patients (23–56%) have uncontrolled persistent asthma.3,4 This group has a high disease burden, with increased exacerbations and hospitalisations, OCS use, Type 2 inflammatory comorbidities and reduced lung function, and lower health-related quality of life.5-13
Impaired Lung Function: What Does It Mean for the Patient?
Professor Marc Humbert
Healthcare professionals often focus on asthma control and exacerbation, but future risk linked to OCS use and lung function decline should also be considered. GINA guidelines recognise the importance of lung function assessment in the personalised asthma management cycle: assess (including lung function), adjust, and review response (including lung function).14 Impaired lung function forms part of the GINA and ERS/American Thoracic Society (ATS) criteria for uncontrolled asthma.14,15 The checklist also encompasses frequent OCS use, serious exacerbations, and poor health-related quality of life.
There are a number of ways to assess persistent airflow limitation. Spirometry measures are the most commonly used: pre or post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1), pre-BD FEV1/forced vital capacity (FVC), and pre-BD forced expiratory flow at 25–75% FVC (FEF25–75%).14,16,17 Improvements of 100–200 mL in FEV1 are likely to be clinically important for patients.17 Radiology with CT or X-rays14 can be used to assess the parenchyma and dilatation of the lungs, but excessive use should be avoided.
The advent of new therapies has brought marked improvement in lung function in patients at a fixed degree of FEV1.18 A published case of a 39-year-old female with uncontrolled severe asthma, who had previously failed on several biologics, showed that after 5 months of dupilumab 300 mg every 2 weeks, asthma control improved, mucous plugging reduced, and airway hyper-responsiveness was also reduced.18 In addition, levels of Type 2 biomarkers, such as FeNO and EOS, declined and ventilation normalised (measured using hyperpolarised helium-3 MRI).18
Current asthma control is clearly important, but how does asthma impact lung function over time? Natural decline in lung function begins in early adulthood but is substantially larger in
people with asthma.19 A 15-year follow-up study showed that the average decline in FEV1 was greater in patients with asthma versus those without asthma (38 mL/year versus 22 mL/year, respectively).19 In a study of patients with persistent childhood asthma (N=684), 75% experienced abnormal lung growth or lung function decline during early adulthood.20 Preserving, or at least improving, lung function is not a pipe dream. The Phase III Liberty Asthma Quest trial showed that dupilumab achieved fast and sustained improvement in post-BD FEV1 over 52 weeks, compared with a 40 mL loss of lung function observed with placebo.16,21
Impaired lung function is clinically important. Low FEV1 in asthma is a strong independent predictor of exacerbations14,22 and recent exacerbations increase risk of future exacerbations.23 This creates a vicious circle. On top of this, severe exacerbations correlate with progression of persistent airflow limitation24 and further increase the rate of lung function decline.24 Data in 1,865 patients aged ≥18 years with moderate-to-severe asthma with 36-month follow-up show a stepwise increase in exacerbation rate with increasing severity of lung dysfunction.22 Compared to those with normal FEV1 at baseline (≥80%), those with mild-to-moderate FEV1 (50–79%) had a 19% higher risk of severe exacerbation over 36 months (p<0.01) and those with severe FEV1 (<50%) had a 59% greater risk (p<0.0001).22
Exacerbations beget exacerbations. In patients ≥12 years enrolled in the TENOR study (N=2,780), the unadjusted odds ratio of future severe exacerbations associated with recent severe exacerbations was 6.33 (95% confidence interval [CI]: 4.57–8.76). Risk remained raised after adjusting for GINA severity, baseline demographic and clinical characteristics, and other factors.23 Clinically, this means that by reducing the number of severe exacerbations, lung function should be preserved.
The frequency of exacerbations is also relevant, with greater frequency associated with a faster rate of lung function decline. In adult patients with moderate or severe persistent asthma followed for at least 5 years (median follow-up of 11 years), the estimated annual decline in FEV1 (mL/year) was 14.6 mL/year in those with <0.1 exacerbations per year, compared to 31.5 mL/year in those with >0.1 exacerbations per year (p<0.05).25 Similarly, in patients with asthma aged 5–66 years, the mean 3-year decline from baseline in post-BD FEV1 (% predicted) was 2.4% in those with no severe exacerbations during the 3-year period, and 6.4% in those with ≥1 severe exacerbations (p<0.001).8 Therefore, it appears that there is crosstalk between FEV1 and exacerbations: low FEV1 increases the risk of exacerbation and exacerbation leads to FEV1 decline.
There are now good data showing that severe asthma exacerbations correlate with the progression of irreversible airflow limitation. In a 3-year follow-up study of 128 patients with well-controlled asthma at baseline, decline in FEV1 was more pronounced as the number of exacerbations rose.24 In addition, BD reversibility was more preserved in patients with no exacerbations but declined in those with exacerbations.24 Taken together, the findings mean that asthma exacerbations could have long-term adverse consequences on airway structure and function.
Reduced FEV1 is also associated with an increased risk of mortality. A 25-year follow-up in adult patients with asthma (N=1,075) showed that the relative risk of all-cause mortality compared to those with normal baseline FEV1 (≥80%) was 2.9 (95% CI: 1.2–3.7) in those with mild-to-moderate FEV1 (p<0.001), and 4.8 (95% CI: 1.6–7.1) in those with severe FEV1 (p<0.001).26
Patients may not recognise that their lung function is reflected in their quality of life. It is important to bear in mind how someone feels, functions, and survives. Qualitative research describes how impaired lung function substantially impacts quality of life due to wheezing, coughing, trouble breathing, panic, a feeling of suffocation, and fear of dying.27
The good news is that improved lung function is correlated with improved health-related quality of life. A retrospective analysis of patients with uncontrolled persistent asthma consisting of 27 randomised studies and 8,994 patients found that improvement in % predicted FEV1 (baseline: 67.10%; Week 12: 74.70%) was moderately correlated with a rise in Asthma Quality of Life Questionnaire (AQLQ) scores (baseline: 4.50; Week 12: 5.25; r: 0.38; p<0.001).28 A small prospective study (N=51) showed that improved lung function was also correlated with increased physical activity.29
The Patient Understanding Leading to Assessment for a Severe Asthma Referral Group (PULSAR) has produced a patient checklist for self-assessment of severe asthma.30 Patients are asked to tick ‘clear signs’ and ‘concerning signs’ of severe asthma.30 This could be a useful exercise to perform in the waiting room as an indicator of improved future management.
Many patients with asthma have comorbid Type 2 inflammatory conditions that impact their lung function and quality of life. For example, anecdotally, patients report that chronic rhinosinusitis with nasal polyps (CRSwNP) is linked with poor sleep and lack of sense of smell. Asthma with comorbid CRSwNP is associated with lower lung function9 and greater annual decline in lung function.31 Type 2 comorbid conditions raise the risk of asthma exacerbations. This was demonstrated in the Optimum Patient Care Research Database (OPCRD) of 118,981 asthma patients in which those with rhinitis or nasal polyps had a higher likelihood of exacerbations.32 Similarly, the Severe Asthma Research Program (SARP) 3 database of 709 patients with asthma found that the annual rate of exacerbations in those with comorbid sinusitis was 4.4 (95% CI: 3.1–6.2) per year, compared to 2.5 per year (95% CI: 1.8–3.6) in those without sinusitis (p<0.001).33
GINA guidelines state that Type 2 inflammation is present in approximately 50% of patients with severe asthma.14 This has been confirmed in a number of studies. In the Belgian Severe Asthma Registry (BSAR) (N=350), 57% of patients with severe asthma had Type 2 inflammation (elevated blood/sputum EOS or FeNO).34 Similarly, in the Phase II lebrikizumab study (N=219), 51% of patients with severe asthma had Type 2 inflammation (elevated serum IgE or EOS).35 The rate of Type 2 inflammation (elevated blood EOS or FeNO) was 71% in a retrospective study (N=200) of patients with difficult asthma, despite high-dose ICS.36 While in the Phase III dupilumab study (N=1,902) of patients with moderate-to-severe asthma, 80% had Type 2 inflammation (elevated blood EOS or FeNO).21 Elevated levels of these Type 2 inflammatory biomarkers are associated with greater disease burden.
In a study of 406 patients, increased bronchial hyper-responsiveness was found in those with elevated EOS, FeNO, or both.37 A study of 610 patients showed greater severe exacerbation rates in patients with elevated FeNO and EOS compared to normal controls.38
Unravelling Type 2 Inflammation and its Role in Lung Physiology
Professor Klaus F. Rabe
Type 2 inflammation is an umbrella term used to categorise certain types of inflammation. There are a number of clinical biomarkers of Type 2 inflammation; the GINA criteria for Type 2 inflammation are blood EOS ≥150 cells/μL, and/or FeNO ≥20 parts per billion (ppb), and/or sputum EOS ≥2%, and/or allergen driven asthma, and the need for maintenance OCS.14 Elevated FeNO, EOS, and IgE are driven by the activity of the cytokines IL-4/13 and IL-5, which can be produced by the adaptive immune system on recognition of allergens.14,34,39-46 In addition, Type 2 inflammation may be accompanied by atopy.14,45
Thus, what roles do IL-4/13 and IL-5 play in Type 2 inflammation and asthma pathophysiology? When an inhaled allergen or other irritant approaches the mucosal surface of the airway epithelium, a signal transduction mechanism transports this information to the lower surface of the mucosa. Dendritic cells form a response to the antigen while thymic stromal lymphopoietin, IL-25, and IL-33 correspond directly with the epithelial surface. The formation and activation of dendritic cells leads to T-cell differentiation, giving rise to IL-4/13 and IL-5.14,40,44,46
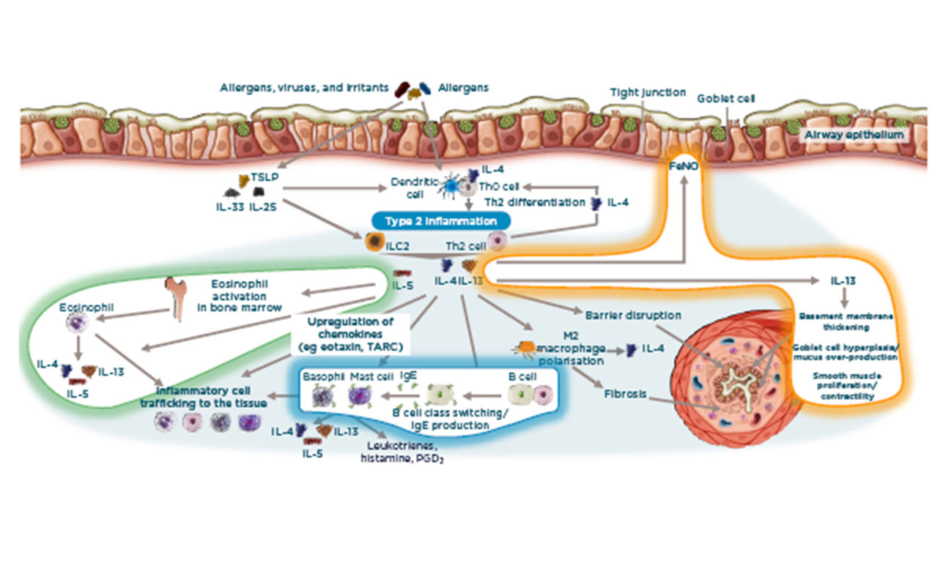
Overall, there are three important areas in the Type 2 inflammation concept (Figure 1). Firstly, EOS-driven inflammation occurs, which involves the cell itself being recruited, activated, and then surviving. Here, IL-5 recruits EOS from the bone marrow to central organs such as the lungs, this is vital because EOS require IL-5 to survive. Secondly, IL-4/13 are relatively upstream (i.e., very close to the epithelium) and are activators of, for example, the change of B cells to IgE-producing B cells. IL-4 acts on mast cells, which themselves produce IL-4/13 and IL-5. Consequently, there is an autocrine loop whereby these cells are implicated in the cellular response, producing IgE, and producing more IL-4/13 in the cell. Regarding lung function, IL-4/13 directly affect the airway structure. This involves basement membrane thickening, impaired epithelial integrity, and proliferation of M2 macrophages, which directly contributes to changes such as fibrosis. Finally, there is a direct loop from IL-4/13, making the biomarker FeNO. 14,40,44,46
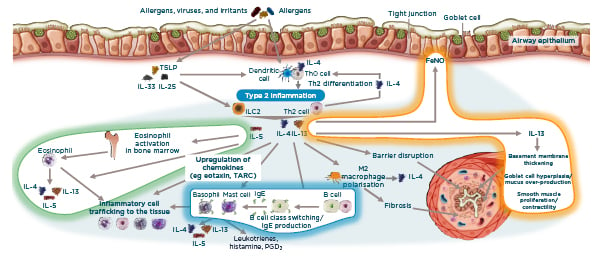
Figure 1: IL-4/13 and IL-5 are key cytokines in asthma pathophysiology.14,40,44,46
FeNO: fractional exhaled nitric oxide; ILC2: innate lymphoid cells; PGD2: prostaglandin D2; TARC: thymus and activation regulated chemokine; TLSP: thymic stromal lymphopoietin.
As IL-4/13 and IL-5 play key roles in Type 2 pathophysiologic effects, they subsequently have effects on the clinical signs and symptoms of asthma (Figure 2).14,40,40,47 The contribution of each cytokine to Type 2 pathophysiology is different: IL-5 is primarily an EOS driver, therefore the biomarker for this is eosinophilia and a drop in EOS is observed in patients. IL-4/13 have a kaleidoscope of actions within the airway, from inflammation, to airway structure, to obstruction; therefore, these cytokines are targeted.
Figure 2: Key Type 2 cytokines IL-4/13 and IL 5 in the clinical manifestations of asthma.14,40,44,47
ILC2: innate lymphoid cells; PGD2: prostaglandin D2; QoL: quality of life.
Type 2 inflammation underpins lung pathophysiology in asthma.44,48 For example, environmental and host factors lead to epithelial barrier dysfunction and barrier leak, thereby triggering inflammation and production of IL-4/13 and IL-5.44,49,50 These cause persistent inflammation, goblet cell hyperplasia and mucus overproduction, airway remodelling, airway smooth muscle proliferation and contractility, and airway hyper-responsiveness. Clinically, this manifests as exacerbations or asthma attacks, impaired lung function, and symptoms such as wheeziness, chest tightness, and shortness of breath.
In addition to affecting the integrity between goblet cells, cytokines influence their function. Patients with fatal asthma have increased mucous production,48 which is associated with decreased lung function.51 Type 2-linked cytokines contribute to this process: mucous plugging is associated with increased IL-13 and IL-5 gene expression,51 while IL-13 increases MUC5AC gene expression.52 Smooth muscle proliferation and increased contractility contribute to airway obstruction and narrowing.48,53 IL-13 directly increases proliferation of airway smooth muscle and significantly increases calcium influx in response to histamine.53,54 Finally, subepithelial fibrosis is associated with asthma severity and reduced lung function.55-57 IL-13 influences fibrosis directly and indirectly through TGF-β.58,59
Targeted Strategies for Improving Lung Function in the Management of Severe Asthma
Professor Michael Wechsler
Understanding of asthma has evolved significantly over the last few decades. Clinical practice has moved on from solely using bronchodilators to addressing inflammation. Clinicians now acknowledge the importance of identifying the type of asthma (eosinophilic, allergic asthma, Type 2-mediated or non-Type 2), and that this is a dynamic process.
Five targeted biologic therapies have entered the market, enabling a precision approach to treatment. Omalizumab60 (anti-IgE) was introduced for allergic asthma in 2005, mepolizumab61 (anti-IL-5) for eosinophilic asthma in 2015, reslizumab62 (anti-IL-5) for eosinophilic asthma in 2016, benralizumab63 (anti-IL-5Rα) for eosinophilic asthma in 2018, and dupilumab64 (anti-IL-4Rα blocking IL-4/13) for asthma with Type 2 inflammation in 2019.
GINA guidelines recommend considering add-on biologic Type 2-targeted treatments for patients with exacerbations or poor symptom control on a high-dose ICS–long acting beta agonist, who either have allergic disease, eosinophilic biomarkers, or need maintenance OCS.14
Which biologic should be started first? The guidelines list eligibility criteria, noting that if one drug is ineffective, another strategy should be considered.14 Patients with skin prick test positivity, IgE levels consistent with allergic inflammation, and exacerbations in the last year are eligible for anti-IgE therapy for severe allergic asthma. Factors that may predict good response to such therapies are blood EOS ≥260 cells/μL, FeNO ≥20 ppb, allergen-driven symptoms, and childhood-onset asthma. Patients with exacerbations in the last year and blood EOS ≥300 cells/μL are eligible for anti-IL-5/anti-IL-5R therapy for severe eosinophilic asthma. In this situation, factors that may predict good response are higher blood EOS, more exacerbations in the previous year, adult-onset asthma, and CRSwNP/nasal polyposis. Patients are eligible for anti-IL-4R therapy if they have severe eosinophilic/Type 2 asthma (with exacerbations in the last year and blood EOS ≥150 cells/μL or FeNO ≥25 ppb), or if they need maintenance OCS. Factors that may predict good response to anti-IL-4R are higher blood EOS and higher FeNO.
What is the effect of these Type 2-targeted biologics on lung function, exacerbations, and patient reported outcomes? In patients with moderate-to-severe allergic asthma, omalizumab reduced exacerbations by 33–75%, but had a modest effect on lung function (3% improvement in mean pre-BD FEV1 percentage predicted compared to placebo; p<0.05).65 However, omalizumab provided significantly greater improvements versus placebo in asthma related quality of life and asthma symptom scores at the end of a corticosteroid reduction phase.66
In patients with severe eosinophilic asthma, treatment with mepolizumab 75 mg intravenously or 100 mg subcutaneously improved lung function by 180–186 mL over 32 weeks compared to baseline; against placebo this was a 100 mL improvement (p<0.05).67 Compared to placebo, asthma exacerbations reduced by 47–53% with the two dosing strategies (p<0.001).67 Mepolizumab also provided a significantly greater improvement in quality of life relative to placebo.67
In patients with inadequately controlled eosinophilic asthma (EOS ≥400 cells/μL), reslizumab demonstrated a 201–235 mL improvement in lung function at Week 52 compared to baseline, translating to a 90–130 mL benefit over placebo (p<0.01; p<0.0001, respectively).68 Asthma exacerbations also reduced by 50–59% relative to placebo (p<0.0001).68 In addition, reslizumab provided a significantly greater improvement in quality of life compared to placebo.68
In patients with severe eosinophilic asthma (EOS ≥300 cells/μL), benralizumab improved lung function by 330–398 mL from baseline to 48–56 weeks in the SIROCCO69 and CALIMA70 studies. The change compared to placebo was 110 mL (p<0.05) and 160 mL (p<0.001) with benralizumab every 4 or 8 weeks, respectively, in SIROCCO69 and 130 mL (p<0.01) and 120 mL (p<0.05) with benralizumab every 4 or 8 weeks, respectively, in CALIMA.70 Additionally, there was a variable reduction in exacerbations: 45–51% (both p<0.0001 versus placebo) in SIROCCO,69 and 36% (p<0.01) and 28% (p<0.05) in CALIMA.70 Benralizumab every 8 weeks provided significantly greater improvements in asthma related quality of life and asthma control relative to placebo.69,70
In patients with uncontrolled, persistent asthma, dupilumab demonstrated a 320–340 mL increase in pre-BD FEV1 from baseline to Week 12, which was sustained through to Week 52.21
Dupilumab provided significant improvements in lung function compared to placebo, observed as early as Week 2 and sustained through 52 weeks of treatment (p<0.001).21 Lung function at Week 12 was also analysed in a number of subgroups administered dupilumab.21,71-73 In patients with EOS >150 cells/μL, there was a 360–370 mL improvement from baseline to Week 12 with the 200 mg and 300 mg dose of dupilumab, respectively, translating to 170 mL and 150 mL improvements compared to placebo, respectively (p<0.001). The magnitude of improvement increased in patients with EOS >300 cells/μL: 430–470 mL improvement from baseline to Week 12 with the 200 mg and 300 mg dose of dupilumab, respectively, translating to 210 mL and 240 mL improvements compared to placebo, respectively (p<0.001).
Looking at subgroups according to FeNO, those with FeNO >25 ppb had 440–450 mL improvement from baseline to Week 12 with the 200 mg and 300 mg dose of dupilumab, respectively, translating to 230 mL and 240 mL improvements compared to placebo, respectively (p<0.001). Considering subgroups of patients with more severe disease, those with >2 exacerbations in the prior year had 340–390 mL improvement from baseline to Week 12 with the 200 mg and 300 mg dose of dupilumab, respectively, translating to 170 mL improvements compared to placebo with both dosages (p<0.0001). Patients with >3 exacerbations in the prior year had 330–400 mL improvement from baseline to Week 12 with the 200 mg and 300 mg dose of dupilumab, respectively, translating to 160 mL (p<0.01) and 220 mL (p<0.0001) improvements compared to placebo, respectively. Finally, the subgroup of patients with airflow obstruction as measured by FEV1/FVC <0.7 and late-onset asthma had 340 mL improvement from baseline to Week 12 with both the 200 mg and 300 mg dose of dupilumab, translating to 150 mL and 200 mL improvements compared to placebo, respectively (p<0.01).
Turning to airway obstruction and small airway function, dupilumab demonstrated significant benefits in pre-BD FEV1/FVC ratio and pre-BD FEF25–75% relative to placebo, which were sustained over 52 weeks (all p values <0.05).16 Looking at exacerbations, patients with EOS >150 cells/μL had a 56% and 60% reduction in adjusted annualised severe asthma exacerbations with the 200 mg and 300 mg doses of dupilumab compared to placebo, respectively (p<0.001).21,71 Those with EOS >300 cells/μL had an even greater benefit: 66% and 67% reduction in adjusted annualised severe asthma exacerbations with the 200 mg and 300 mg dose of dupilumab compared to placebo, respectively (p<0.001).21,71 Meanwhile,
patients with FeNO >25 ppb had 65% and 61% reductions in adjusted annualised severe asthma exacerbations with the 200 mg and 300 mg dose of dupilumab compared to placebo, respectively (p<0.001).21,71 On top of the benefit to exacerbation rate, dupilumab provided significantly greater improvements in quality of life and asthma control relative to placebo.21,71
In the last couple of years, the Asthma & Allergy Network and GINA have emphasised the need to eliminate chronic maintenance OCS use.14,74 Several of the biologics have demonstrated improved lung function and reductions in exacerbations while reducing OCS. Mepolizumab reduced asthma exacerbations by 32% compared to placebo (p<0.05) while tapering OCS dose by 50%.75 In the ZONDA study of patients dependent on OCS and with EOS >150 cells/μL, those on placebo had a 25% reduction in OCS dosing while benralizumab-treated patients had a 75% reduction.76 Despite a much larger reduction in OCS use, there was a 55% (p<0.01) and 70% (p<0.001) reduction in annualised exacerbation rate with benralizumab every 4 or 8 weeks compared to placebo, respectively.76
In the VENTURE trial, dupilumab achieved an average 70% reduction in OCS dosing over 24 weeks versus 42% with placebo (Figure 3).77,78 Looking at the median, there was a 100% reduction in OCS with dupilumab versus 50% with placebo.77,78 While tapering their OCS use, patients taking dupilumab had a 220 mL improvement in lung function relative to placebo at Week 24 (p<0.001), as well as a 59% reduction in adjusted annualised severe asthma exacerbations compared to placebo (p<0.001).77,78
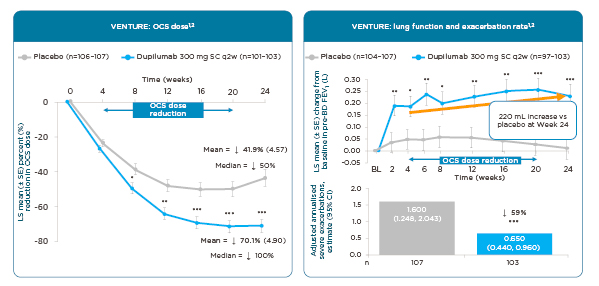
Figure 3: Dupilumab: effect on oral corticosteroid use, lung function, and exacerbations in an oral corticosteroid sparing study (Phase III).77,78
*p<0.05; **p<0.01; ***p<0.001 versus placebo.
BD: bronchodilator; BL: baseline; CI: confidence interval; FEV1: forced expiratory volume in 1 second; LS: least squares; OCS: oral corticosteroids; SC: subcutaneous; SE: standard error; q2w: every 2 weeks; vs: versus.
The findings were corroborated in a real-life study of 64 patients in France with severe asthma, of whom 76% were receiving OCS daily (median dose 20 mg/day), and 84% and 17% had received previous treatment with omalizumab and mepolizumab, respectively.79 After treatment with dupilumab, there was a significant improvement in lung function, with a 450 mL difference at Month 12 compared to baseline (p<0.001), and a 75% reduction in exacerbations over the same time period (p<0.001).79 There was also a significant improvement from baseline to 12 months in asthma control scores.79
Regarding safety, injection site reactions occur in a small percentage of patients: 14% and 18% with the 200 mg and 300 mg doses, respectively, over 6 months compared to 6% with placebo. During the same period, oropharyngeal pain occurred in 2% of patients on both doses (versus 1% with placebo), and the same for eosinophilia (compared to less than 1% with placebo).80
The ERS symposium is available for replay via adventprogram.com, an educational site developed in collaboration with world-renowned experts in Type 2 inflammatory diseases including atopic dermatitis and asthma and provided by Sanofi Genzyme and Regeneron.
Learn more about identifying, assessing, and managing asthma with type 2 inflammations at ADVENTprogram.com