Chairpeople: Eric Bateman1
Speakers: Alvar Agusti,2 Anna Murphy3
1. University of Cape Town, South Africa
2. Hospital Clínic de Barcelona, Spain
3. University Hospitals of Leicester, UK
Disclosure: Bateman has acted as a speaker for ALK, AstraZeneca, Chiesi Famaceutici S.p.A., Boehringer Ingelheim, Orion Menarini, Novartis, Regeneron Pharmaceuticals, and Sanofi Aventis; and provided consultancy for ALK, AstraZeneca, Novartis, Regeneron Pharmaceuticals, and Sanofi Aventis. Murphy has received research funding, consultancy fees, or honoraria for presentations from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici S.p.A., GlaxoSmithKline (GSK), Orion, Sanofi, and Trudell Medical International. Agusti has received research grants from AstraZeneca, GSK, and Menarini Group; lectured for AstraZeneca, Chiesi Farmaceutici S.p.A., GSK, Menarini Group, and Orion; and served as member of scientific advisory board for AstraZeneca, Chiesi Farmaceutici S.p.A., GSK, Menarini Group, and Merck Sharp & Dohme (MSD).
Acknowledgements: Medical writing assistance was provided by Ville Vartiainen, Järvenpää, Finland.
Support: The symposium was funded by OrionPharma and the Menarini Group. The views and opinions expressed are those of the authors and not necessarily of funders.
Citation: EMJ Respir. 2021;9[1]:47-51.
Meeting Summary
This symposium took place during the 2021 virtual meeting of the European Respiratory Society (ERS). It focused on improving the management of asthma and chronic obstructive pulmonary disease (COPD) by empowering patients and personalising their treatment. Alvar Agusti discussed the treatable traits of COPD and focused on use of inhaled corticosteroids (ICS). He concluded that, while ICS treatment imposes a slightly increased risk of pneumonia, it decreases all-cause mortality in patients with elevated blood eosinophils. Anna Murphy described inhaler devices and their use. Errors in device use are common and no improvement in the inhaler technique of patients has been made during the modern history of inhaler use. For successful inhaler therapy, personalised choice of the device and continuous training are paramount. Finally, Eric Bateman described the theory and practice of as-needed ICS/formoterol as a mean to empower patients to take responsibility for their asthma management. During his talk, he described the reasoning and evidence that has made as-needed ICS/formoterol the preferred treatment approach in the 2021 Global Initiative for Asthma (GINA) report.
Introduction
Bateman began the symposium by describing patient empowerment. Empowerment is a process through which people gain greater control over decisions and actions affecting their health. They become actively involved in their treatment, instead of being passive objects of medical interventions by healthcare professionals. Patient education can help patients to understand what they can do to affect their own health and promote understanding that patients can be equal partners in their healthcare decisions.
Personalised medicine implies individualised treatments that are available for every unique patient. It must not be confused with precision medicine, which seeks to create treatments that are applicable to groups of individuals who exhibit certain characteristics.
This symposium was concerned with personalised medicine and how patient empowerment and involvement can lead to better disease outcomes.
Personalising Treatment in Chronic Obstructive Pulmonary Disease: The Case for Inhaled Corticosteroids
Alvar Agusti
COPD is a complex and heterogeneous disease. Complex means that it has several elements (e.g., forced expiratory volume, exacerbations, symptom perception, and comorbidities with non-linear relationships), which means that one cannot be predicted from the other. Heterogeneous means that not all these elements are present in all patients, and they may even vary over time in the same patient, either because disease improvement by treatment or disease progression.
To address this complexity and heterogeneity, a phenotypic-based strategy was proposed back in 2010.1 However, it was later realised that this was a too simplistic approach, since patients often exhibit several so-called treatable traits (TT).2
TTs are not necessarily tied with a specific diagnostic label and can occur in the pulmonary, extra-pulmonary, and behavioural or environmental domains. TTs can be identified either by clinical examination and expertise (phenotypes), or validated biomarkers that inform on the presence of specific biologic mechanisms (endotypes). TTs can coexist and change with time, either in spontaneously or response to treatment.
The goal of treatment with ICS in COPD is to reduce the risk of exacerbations in patients who suffer from exacerbations despite appropriate treatment, with one or two long-acting bronchodilators (LABD).3 This has to be balanced against the increased risk of pneumonia. The level of circulating eosinophils (eos) is a useful biomarker, predicting the response to ICS in COPD4 as the risk of exacerbations increases as function of blood eos count.5-7 The preventive effect of ICS on exacerbation is higher in patients with higher circulating blood eos, particularly above 300 eos/µL; below 100 eos/µL, when ICS are no different from the use of LABD; and the risk of pneumonia increases below 100 eos/µL.8 Finally, the IMPACT9 and ETHOS10 studies showed a significant reduction in all-cause mortality when ICS were added to LABD in patients with frequent exacerbations, despite their use.
Collectively, these observations indicate that ICS treatment in patients with COPD must be individualised. As shown below, ICS must be used thoughtfully.3 There is strong support for their use if there is history of hospitalisations for exacerbations of COPD, 2 or more moderate exacerbations of COPD per year, a blood eos count of >300 cells/µL, or there is concomitant asthma (Table 1). The use of ICS should be considered individually if patients have between 100–300 eos/µL or in patients with a moderate exacerbation. Finally, ICS are not recommended in patients with repeated pneumonia events and/or those with less than 100 eos/µL.
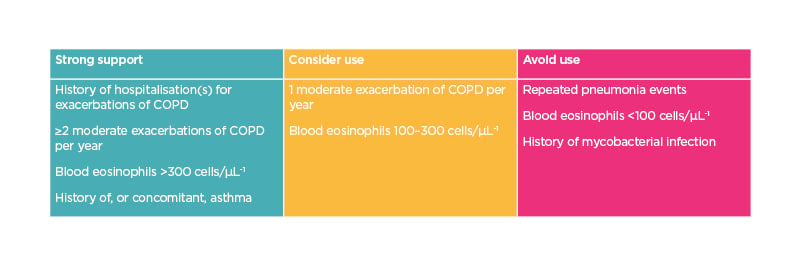
Table 1: Recommendations on use of inhaled corticosteroids in chronic obstructive pulmonary disease based on blood eosinophil count.
COPD: chronic obstructive pulmonary disease.
Selecting the Right Device for Personalised Management of Asthma and Chronic Obstructive Pulmonary Disease
Anna Murphy
Murphy discussed importance of optimising inhaled medication delivery, personalising the choice of delivery device, monitoring the correct inhaler technique, and the sustainability of inhaler medication. Different delivery devices can be categorised into five different groups: pressurised metered-dose inhaler (pMDI), breath-actuated pMDIs, dry powder inhalers (DPI), nebulisers, and soft mist inhalers. Each inhaler type has different attributes and the choice is determined by both the medication and patients ability and willingness to use the device (Box 1).11

Box 1: Factors affecting the choice of inhaler type.
pMDI: pressurised metered-dose inhaler.
Studies have shown that errors in inhaler technique are common. Although studies are difficult to compare, estimates of inhaler errors include up to 90% of the patients using pMDIs and up to 54% of the patients using DPIs.12-18 Many clinical studies have shown inhaled medication to have excellent safety and efficacy profiles but, in real life, it may be difficult to guarantee that the devices are used correctly. Sanchis et al. reviewed over 100 studies for acceptable inhaler technique and found out that only approximately 40% of patients use their devices correctly and it has remained stagnant for the 40-year history of modern inhaler therapy.17 It is obvious that both healthcare professionals and patients need more training in the use of inhaler devices. Poor inhaler technique has significant association to clinical outcomes of asthma such as exacerbations, asthma control test (ACT) scores, or GINA symptom control measures, irrespective to the country, device type, or age of the patient.19 Poor inhaler technique also inflicts considerable economic burden. In a study conducted in UK, Sweden, and Spain on patients using Turbuhaler® or Accuhaler®, the direct annual costs of poor inhaler technique were 2.2–7.7% of the total costs of asthma, amounting to 105 EUR across the three countries.20 Both the GINA and Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines stress the importance of the individual inhaler and sufficient training.
According to CRITIKAL study inhalation flow error was the most common type and was most prevalent with patients using pMDIs.21 With pMDIs, patients tend to inhale too forcefully, promoting oropharyngeal deposition. This will reduce the efficacy of the medication, while possibly increasing adverse effects.22,23 Another common error was not tilting the head correctly to straighten the airways before inhalation.21
With DPIs, the drug has to be detached often from lactose particles.24 There is a persisting misconception that with DPI devices the airflow resistance may be too high for some patients. In fact, the devices with high internal resistance require much lower inspiratory flow rate to operate. The severity of obstruction does not limit the use of high resistance devices. This is shown by number of studies. For example, when Jõgi et al. studied 100 patients with COPD and 100 healthy volunteers; practically all of the subjects were able to generate sufficient flow rate for the high resistance devices.25 Haughney et al. studied 994 adult patients with asthma and 94% of the patients were able to generate sufficient flow rate with the highest resistance setting of In-Check Dial, corresponding to high resistance DPI; however, 30% of patients failed to correctly use pMDI, inhaling too fast, even after careful tutoring and guidance.26 Patients, using many different kind of devices, are more prone to inhaler errors and achieve worse disease control than those using only one type of device.22,27-29
Lately, there has been lively discussions on sustainability of inhaler treatment. As nearly all of the patients are able to use whichever device they like, sustainability of the inhalers has also become an important factor in the inhaler selection. Particularly, pMDIs have a high carbon footprint due to their propellants. DPIs have been suggested as an alternative when clinically feasible, as their carbon footprint is marginal compared to that of pMDIs. Favouring greener inhalers has been one of the promoted actions by the UK National Health Service (NHS) in their programme towards net zero carbon footprint of health services.
VIDEO RECORDINGS OF THE PRESENTATIONS ARE AVAILABLE FROM WEHALE.LIFE. https://www.wehale.life/events/








