Abstract
Background: During the recent coronavirus disease (COVID-19) pandemic there have been several studies implicating an association between obesity, COVID-19 severity, and mortality. This retrospective study aims to investigate the association between obesity, other risk factors, and COVID-19 mortality of patients admitted over a 6-week period to the respiratory units at the authors’ hospitals.
Methods: This is a retrospective study of 71 patients who were admitted into a respiratory unit over a 6-week period where the data were analysed for correlation between various risk factors, COVID-19 severity, and mortality. The statistical analysis was performed using excel statistics and SPSS (IBM, Armonk, New York, USA) statistical software. The significance was considered at p<0.05. The multivariate analysis, Z-test, Cox regression, Pearson correlation, and Kaplan–Meier analysis were used.
Results: The mean age of the patients was 65.8 years (range: 35.0–93.0 years) standard deviation (13.21) and the male to female ratio was 2.73 (52:19, respectively). The most frequent comorbidities were obesity (42/71; 59%), hypertension (36/71; 50%), diabetes (22/71; 31%), heart disease (13/71; 18%), respiratory disease (9/71; 13%), and cancer (8/71; 11%). The mean body weight was 83.7 kg (60.4–147.7 kg) and the mean BMI was 32.2 (22.0–53.0 kg/m2). Smoking was reported in 8 (11%) of the patients. There were 20 (83%) mortalities among patients >70 years old (p<0.0001), 20 (83%) deaths among male patients (p<0.0001), 14 (58%) deaths among patients with a BMI >25 kg/m2 (p=0.001), 17 (70%) deaths reported for patients with hypertension (p=0.008), 6 (25%) mortalities for patients with cardiovascular disease (p=0.001), 14 (30%) deaths among patients who were mechanically ventilated (p=0.00028), and 5 (20%) mortalities among patients with cancer (p=0.003).
Conclusions: Obesity, cancer, mechanical ventilation, male sex, intensive care unit admission, cardiovascular disease, and hypertension are significant risk factors for mortality in patients with COVID-19.
INTRODUCTION
Obesity is suggested as a risk factor for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection,1,2 though this has not been confirmed in randomised trials. In the outbreaks of the influenza virus H1N1 and SARS, obesity was reported in 23% of patients and was a predictor and risk factor for severe disease and poor outcomes.3-5 This affinity is based on several underlying factors, mostly related to a defective immune system, antigen presentations, cellular and humoral responses, cytokines activation, interleukins interactions, immune system regulation, and adipose tissue inflammation.6-10 According to the Intensive Care Units National Audit and Research Centre (ICNARC), once the infection is established in patients who are obese, it leads to severe outcomes in the majority.11 The ICNARC data has shown that >73% of newly diagnosed cases of coronavirus disease (COVID-19) had a BMI >25 kg/m2, 74% of patients needed advanced respiratory support, and nearly 50% died in an intensive care unit. The health systems in Western countries with high rates of obesity, including the UK, have experienced challenges whilst managing patients with COVID-19. Despite the huge resources and well-developed health infrastructures, the progression to organ failure and death in a significant number of patients, unfortunately, has been confirmed.12,13 The primary endpoint of this article was to investigate obesity as a risk factor for mortality in COVID-19 patients. The secondary endpoints were to assess age, sex, diabetes, cancer, hypertension, smoking, and respiratory and cardiovascular diseases as risk factors for COVID-19 mortality.
METHODS
Study Design
This was a retrospective study of 71 consecutive patients with COVID-19 who were admitted and treated at a respiratory unit (RU) at the authors’ hospitals. The data was initially retrospectively collected but subsequently populated prospectively during the hospital stay and then analysed. All patients showed severe symptoms of COVID-19 and tested positive for SARS-CoV-2 on a reverse transcriptase–PCR assay after a nasopharyngeal swab. All 71 patients (age range: 35–93 years) were admitted to the RU with hypoxaemia.
Setting
In the university hospitals, the RU was managed and supervised by a consultant respiratory physician. Patients were admitted to the intensive therapy unit (ITU) if they failed respiratory management.
Data Collection
The data were collected by the respiratory and ITU teams over a 6-week period. Initially retrospectively, then prospectively, the data were collected as the patient’s clinical condition developed. The data were collected from case notes and clinical electronic systems; they were saved on Microsoft Excel files with a secured, encrypted password that could only be accessed by the research team. The anonymous data were then passed over to another research team to analyse.
Variables
The variables included age, sex, BMI, smoking, respiratory and cardiovascular disease, hypertension, diabetes, and cancer. The mortality was calculated for each of these risk factors. The data source was the daily patient records from the RU and ITU.
Bias
This is a retrospective study of a cohort of patients; there was no selection bias as all patients were included over the 6-week study period. The clinical decisions were governed by clinical guidelines and supervised by experienced consultant physicians.
Quantitative Variables
The most important demographic features, comorbidities, and mortality rates were reported. The data were added to the Microsoft Excel file as they emerged, which was frequently both during admission and during progression of the disease.
Statistical Analysis
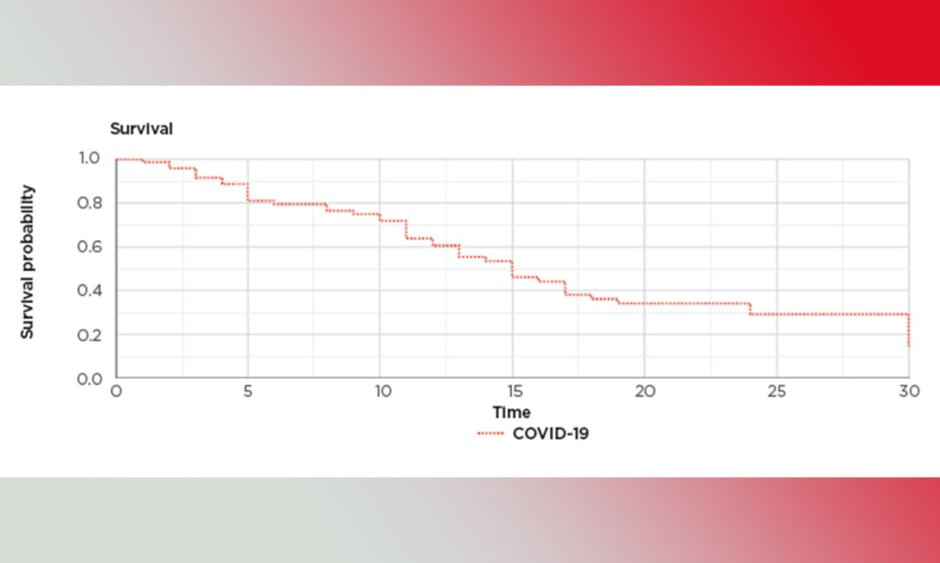
Microsoft Excel statistics and XLSTAT (Addinsoft, Paris, France) statistical software was used. The mean was used to compensate for missing data related to smoking and BMI. The rest of the data for all patients were available and there was no loss to follow-up. The significance was considered at p<0.05. Z-test, multivariate analysis, logistic regression, and correlation tests were used. Cox regression analysis was used to assess the survival and calculate the proportional hazard ratios. A Kaplan–Meier survival graph was produced to show the survival pattern (Figure 1).
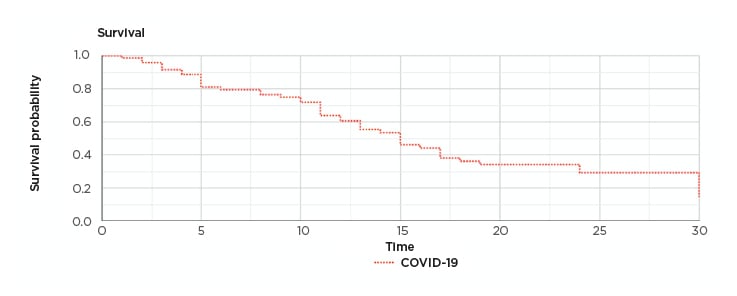
Figure 1: Kaplan–Meier survival graph.
COVID-19: coronavirus disease.
RESULTS
All of the patients’ data were included at all stages of the study. The mean age of the patients was 65.8 years (range: 35–93 years) and the male to female ratio was 2.73 (52:19). The most frequent comorbidities were diabetes (22/71; 31%), heart disease (13/71; 18%), hypertension (36/71; 50%), respiratory disease (9/71; 12.7%), and cancer (8/71; 11%).
The mean body weight was 83.7 kg (60.4–147.7 kg) and the mean BMI was 32.2 (standard deviation [SD]: 6.8; range: 22.0–52.0 kg/m2). Seventeen patients (24%) were managed at Level 2 of care, while 54 were managed at Level 3 of care. The mean number of days needed by the patients to achieve >40% fraction of inspired oxygen (FiO2) was 1.09 days (SD: 1.23). The mean O2 after 12 hours of starting continuous positive airway pressure (CPAP) was 10.35 L/min (SD: 4.38); the partial pressure of oxygen before CPAP (SpO2 pre-CPAP) was 93.60% (SD: 0.04); the FiO2 (last pre-CPAP) was 69.80% (SD: 0.13); the SPO2/FiO2 ratio pre-CPAP was 1.40 (SD: 0.37); the mean respiratory rate pre-CPAP was 24.68 breaths per minute (SD: 4.97); the mean days on CPAP was 3.71 (SD: 2.23); and the mean maximum positive end-expiratory pressure (PEEP) was 12.78 cmH2O (SD: 2.27).
Of the 71 patients, 25 (35%) were discharged, while 46 (65%) were intubated and managed at Level 3 of care.
Twenty-eight patients (39%) were mechanically ventilated. Ten patients (14%) died in the RU and the total mortality up to the date of writing this report was 24 (34%). At present, 16 patients (23%) are still in hospital. The mean length of stay was 10.3 days (SD: 4.9), the mean C-reactive protein level on admission was 147.30 mg/L (SD: 99.93), the peak C-reactive protein was 264.42 mg/L (SD: 113.99), and the D-Dimer mean was 9,742 ng/mL (range: 224–80,000 ng/mL; SD: 22515) (Table 1). The incidence of obesity in this cohort of patients was 59%. The length of hospital stay ranged from 5 to 19 days (SD: 4.9), excluding the 16 patients still in hospital at the time of writing.
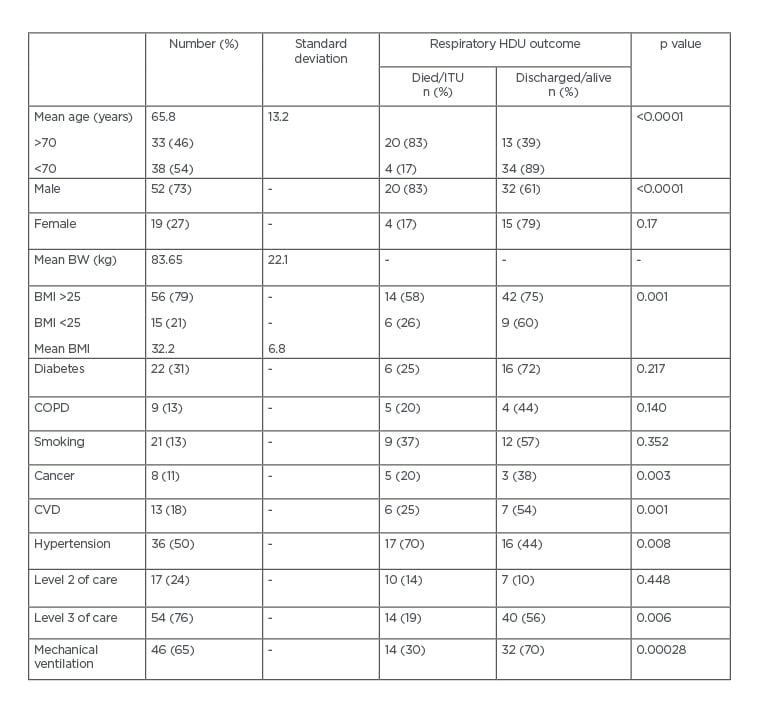
Table 1: Characteristic features and mortality.
BW: body weight; COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; HDU: high dependency unit; ITU: intensive therapy unit.
Multivariate analysis showed a significant association between all the aforementioned risk factors (except chronic obstructive pulmonary disease [COPD] and diabetes; p=0.140 and p=0.217, respectively) and COVID-19 mortality (p=0.000172). There were 20 (83%) mortalities among patients >70 years old (p<0.0001) and 20 (83%) deaths among male patients (p<0.0001). There were 14 (58%) deaths among patients with a BMI >25 kg/m2 (p=0.001), 17 (70%) deaths among patients with hypertension (p=0.008), 6 (25%) mortalities among patients with cardiovascular disease (p=0.001), 9 (37%) deaths among those who smoked (p<0.00001), and 5 (20%) mortalities among patients with cancer (p=0.003). On multi-regression analysis, BMI (p<0.0001), cancer (p=0.003), and smoking (p<0.0001) significantly predicted mortality (Table 1). Cox regression analysis showed an increased proportional hazard ratio for age, heart disease, hypertension, cancer, obesity, sex, and the need for mechanical ventilation (Table 2).
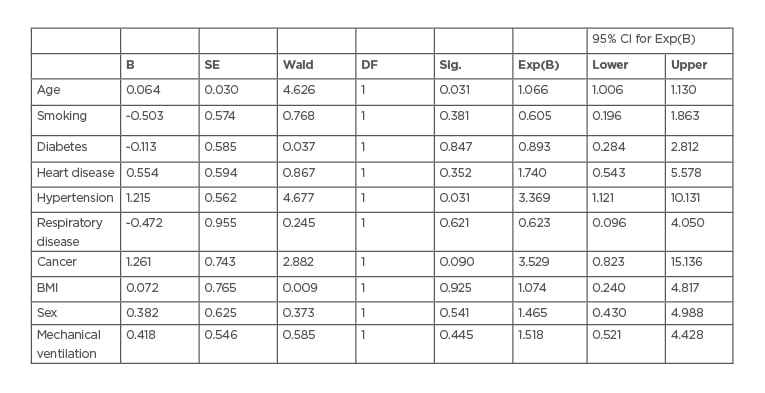
Table 2: Cox regression analysis variables.
B: exponential; CI: confidence interval; DF: Dickley–Fuller test; SE: standard error; sig: significance test; Wald: Wald test.
DISCUSSION
The most striking findings of this study are that obesity is pivotal in severe COVID-19 progression and mortality (p=0.043). Obesity is a complex disease with several factors increasing the likelihood of severe disease and mortality in cases of COVID-19, among them being a local prevalence of obesity; the associated comorbidities of obesity such as diabetes, cardiovascular, stroke, respiratory disease, and defective innate and adaptive immune responses; and the exposure of the patients to the virus because of their frequent hospital visits and need for medical support.14-16 It is not surprising that a significant number of severe COVID-19 cases led to mortality in highly prevalent areas for obesity such as the USA17 and the UK.11
A recent meta-analysis showed that obesity, age, critical illness, the need for advanced respiratory support, and severe comorbidities are risk factors for mortality in patients with COVID-19.18 Obesity is thought to be a major risk factor for COVID-19 mortality because of the impaired immune response, cellular failure to control the infection, and the effect of adipose tissue mass, to which the virus affinity is higher than to the lung tissues.19,20 The overexpression of inflammatory adipokines from visceral fat depots can affect the immune response, impair the chemotaxis, and alter the macrophage differentiation.21 Other publications had confirmed the association between obesity and mortality and leading organisations such as the British Obesity and Metabolic Surgery Society (BOMSS) have called for increasing bariatric surgery capacity to protect patients with obesity and reduce the definitive risks if they go on to develop COVID-19.22
The mean age of the participants was of 65.8 years, and several studies on COVID-19 have confirmed that elderly people are likely to experience the severe form of the infection.23,24 Other important features strongly associated with COVID-19 mortality are male sex, heart disease, respiratory disease, and cancer. Previous studies have also shown the vulnerability of hypertensive patients for severe infection.25
It is known that the virus attaches itself to cells via the angiotensin-converting enzyme-2 (ACE2) receptors for purposes of entry, replication, and subsequent shedding.26 ACE2 are a usual target in the management of hypertension as their receptors are abundant in the lung, heart, kidney, and gastrointestinal tract. Administering ACE2 inhibitors blocks the receptors and reduces angiotensin-1. Angiotensin II receptor blockers are able to reduce inflammation and could diminish the potential for the development of either acute respiratory distress syndrome, myocarditis, or acute kidney injury, which is known to occur in patients with COVID-19.27
Several studies have reported hypertension as an indicator of severity without adjusting the confounding factors. The accurate association is not known and larger studies are needed to address this controversy.28,29 Smoking is frequently reported in cases of COVID-19, and it is a risk for severity, ITU admission, and mortality;30 this is because smoking increases the levels of goblet cells and ACE2 receptors. Theoretically speaking, higher levels of smoking leads to additional ACE2 receptors which gives more opportunity for the SARS-CoV-2 to attach to cells.31 If the viral load is high, then the expected outcome is a severe disease and possible mortality.
It is, however, not clear whether ex-smokers will be at as high a risk as current smokers, and more studies are needed to elucidate this.
Again, confounding factors that need adjustment must be observed before jumping to the conclusion of significance as deemed by statistical tests.
Additionally, cardiovascular disease is a predictor for COVID-19 severity, ITU admission, and mortality. This again is linked to ACE2 receptors and progression to myocarditis, heart failure, and death.32
Patients with COPD are also at significant risk of poor COVID-19 disease progression and mortality. Many other studies have shown similar outcomes in patients with COPD and COVID-19,24,33,34 where significant number of patients were admitted to the ITU or had to undergo CPAP ventilation.35,36
Patients with cancer are especially vulnerable to infection and severe outcomes because of poor general health, immunosuppression, and the type of cancer and anticancer treatment regimen they are following.37,38 Cancer was significantly associated with mortality in the patients in this study (p=0.003).
In this study, diabetes was a predicter of mortality in linear regression; however, it did not reach statistical significance in multivariate analysis (p=0.491). Several reports on patients with diabetes have shown a strong association between vulnerability, severe disease progression, and mortality when presenting with COVID-19.23,39-41 This is likely because of immunosuppression, defective metabolic systems, and associated morbidities of chronic complications of diabetes such as cardiovascular, renal, and cerebrovascular disease.
As this study is relatively small, caution should be taken when considering the generalisability of the results. It is, however, one of several studies to provide evidence on obesity as a risk factor for COVID-19 mortality.
Limitations
Firstly, this is a cohort of consecutive patients who were admitted to the RU for respiratory support and possible escalation to the ITU. It is a relatively small study; however, the sample representation was adequately powered for statistical analysis. Secondly, a significant number of patients are still in hospital and are therefore not available for mortality analysis.
CONCLUSIONS
This is one of several clinical studies to confirm obesity as a risk factor for COVID-19 mortality. Cancer, smoking, cardiovascular disease, and hypertension are also additional risk factors for mortality.