Abstract
Background: COVID-19 is an infectious disease that can infect anyone, including pregnant females, a population that is susceptible to various infections. This has become a challenge because of the risk of vertical transmission and increased foeto-maternal mortality. That authors’ purpose was to present the incidence of pregnancy with COVID-19 and the vertical transmission in pregnancies with COVID-19.
Methods: This study used cross-sectional observational research and was carried out at the Prof. dr. I.G.N.G. Ngoerah Denpasar General Hospital, Denpasar, Indonesia, from January–April 2021. The authors used primary data from pregnant females who presented at the hospital with positive severe acute respiratory syndrome coronavirus 2 PCR results. Additional instruments included data collection forms and medical records.
Results: Based on primary data, a total of 15 pregnant females with COVID-19 were identified from a total of 165 deliveries in that period. The prevalence of COVID-19 events in pregnant females was 9.09%. It was found that the rapid blood antibody results for all infants had non-reactive results for IgM. It can be assessed that the relative risk of transmitting COVID-19 antibodies from mother to foetus is three times (risk ratio: 3.00; 95% confidence interval: 1.56–64.26). One baby was found with reactive examination results so that the prevalence ratio obtained was 11.7 (prevalence ratio: 11.7; 95% confidence interval: 1.63–35.57).
Conclusion: The prevalence rate of pregnant females with COVID-19 at the Prof. dr. I.G.N.G. Ngoerah General Hospital for the period of January–April 2021 was 9.09%. COVID-19 infection in pregnancy can increase the risk of vertical transmission of COVID-19 by 11.7 times compared with pregnancy without COVID-19 infection.
Key Points
1. Despite a decrease in COVID-19 infection rates, infection during pregnancy may result in poor outcomes for some, with a mortality rate of 1.6%, and potential vertical transmission.2. In this study, the authors describe the incidence of pregnancy with COVID-19, along with the vertical transmission of COVID-19, in pregnancies at the Prof. dr. I.G.N.G. Ngoerah Denpasar General Hospital, Denpasar, Indonesia, from January–April 2021.
3. Results indicate that females with COVID-19 who are pregnant have an 11.7 times higher risk of vertically transmitting it to their babies compared with those without COVID-19 infection.
INTRODUCTION
COVID-19 is an infectious disease that has become a worldwide pandemic. This disease can infect anyone who has been exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including pregnant females, who are a population that is more susceptible to various infections due to their immunocompromised state.1,2 Here in lies the challenge. Since there would be the possibility of a different COVID-19 clinical course in pregnant females, with different treatment considerations to the risk of vertical transmission and increased foeto-maternal mortality, studies regarding COVID-19 in pregnant females need to be developed further to ensure the best care possible for this specific population group.3-5
Indonesia has been one of the biggest hotspots for COVID-19, especially in Southeast Asia. As of 15th March 2023, there have been well over 6 million confirmed cases since 2020, and over 160,000 deaths.6 Although epidemiological research on COVID-19 in pregnancy in Indonesia is still quite limited, according to the Routine Family Health Data from the Indonesian Ministry of Health, there has been an increase in the number of maternal deaths during the pandemic in areas with a distribution of COVID-19 cases.7,8
Physiological changes in the immune system of pregnant females have long been known to increase susceptibility to infections in general. Apart from that, the virus is also thought to be able to bind to angiotensin converting enzyme (ACE) 2 receptors in various body tissues, including the placenta, hence allowing it to cross the placental barrier and be transmitted vertically.9
Vertical transmission of SARS-CoV-2 is yet to be formally defined, but Blumberg et al.10 proposed that vertical transmission is detected if the mother is positive for COVID-19 between 14 days before birth and up to 2 days after birth; the virus is detected at the neonate’s respiratory tract or blood sample, amniotic fluid, or umbilical cord blood; and there are signs of persistence from either a positive swab of the respiratory tract after 24 hours of life, or a positive SARS-CoV-2 IgM in the first 7 days of life. Mahyuddin et al.11 also stated that to diagnose transplacental infection, samples taken from the placenta, umbilical cord, neonatal airway, rectum, or amniotic fluid (prior to rupture of membranes) for real-time PCR are needed.
Even though incidence rates of COVID-19 have been decreasing, the authors still consider the fact that COVID-19 infection in pregnancy may result to poor outcomes in some, with a mortality rate of 1.6%, and that it may cause vertical transmission.12-15 Therefore, the authors are interested in further research to provide knowledge about the epidemiology, characteristics, and impacts of COVID-19 infection in pregnancy. In this study, the authors will describe the incidence of pregnancy with COVID-19, along with the vertical transmission of COVID-19 in pregnancies at the Prof. dr. I.G.N.G. Ngoerah Denpasar General Hospital, Denpasar, Indonesia, from January–April 2021.
METHODS
This study used a cross-sectional, observational research method and was carried out at the Central General Hospital Prof. Dr. I.G.N.G. Ngoerah Denpasar from January–April 2021. The reachable population included pregnant females with COVID-19 at this hospital during this period. The inclusion criteria for eligibility were: all obstetrics patients with confirmed COVID-19 from reverse transcription-PCR (RT-PCR) who gave birth at General Hospital Prof. Dr. I.G.N.G. Ngoerah Denpasar from January–April 2021, who have signed the informed consent; mothers who went through Caesarean section delivery; and all babies born from the confirmed COVID-19 mothers. The participants were excluded if the obstetric patient who was about to give birth had a negative RT-PCR result, they had incomplete data, or the mother gave birth vaginally.
The primary data for this study would be the pregnant females who came to Prof. Dr. I.G.N.G. Ngoerah referred from primary or secondary healthcare facilities that brought positive SARS-CoV-2 RT-PCR results. Additional instruments in this study were data collection forms and medical records. The data of this research were obtained from pregnant females with COVID-19 who were about to give birth and patients in the COVID-19 isolation room that were selected according to the research sample criteria, namely patients who were pregnant during the study period.
After the pregnancy of the females who were COVID-19-positive were terminated by Caesarean section, blood samples to test for IgM and IgG anti-SARS-CoV-2 were taken from the newborns to indicate the vertical transmission of COVID-19.
The data taken was entered into the Microsoft Excel computer software (Microsoft, Redmond, Washington, USA). The data that was obtained was then tabulated and analysed to calculate the prevalence of pregnancy with COVID-19 and the prevalence of the vertical transmission ratio of COVID-19.
RESULTS
Based on the primary data of pregnant females with COVID-19 at the Central General Hospital Prof. Dr. I.G.N.G. Ngoerah Denpasar from January–April 2021, a total of 15 pregnant females were positive with COVID-19 from a total of 165 deliveries in that period, so that the prevalence of COVID-19 infection in pregnant females at this hospital for that period was 9.09%. An overview of the prevalence of pregnant females with COVID-19 infection can be seen in Table 1.
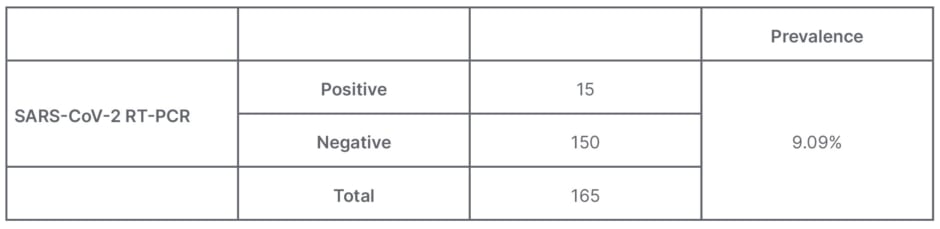
Table 1: Prevalence of pregnancy with COVID-19 infection at the Prof. dr. I.G.N.G. Ngoerah General Hospital, Denpasar, Indonesia.
The serum IgM and IgG findings from the 15 neonates born to mothers with COVID-19 only resulted in one positive result, which was positive only for IgG, but not IgM.
After calculating the prevalence ratio by comparing the positive group with the negative group, it is found that the PR is 11.7% (PR: 11.7; 95% CI: 1.63–35.57).
CI: confidence interval; PR: prevalence ratio; RT-PCR: reverse transcription PCR; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Epidemiological data on maternal cases of COVID-19 are still minimal, but the prevalence from this study is consistent with several studies, which state that the prevalence of COVID-19 in pregnancy ranges from 3–20%.16
In this study, it was found that the rapid blood antibody results for all newborns were non-reactive for IgM. This is because IgM is usually unable to be transferred from mother to foetus due to its large macromolecular structure that is incapable of crossing the intact placental blood barrier.17 These results are different from one study conducted in Wuhan, China, which found that there were newborns born to mothers with COVID-19 who had positive IgM anti-SARS-CoV-2 at birth.18 This reactive IgM result can occur if the virus itself, rather than the IgM antibody, is transmitted through the placenta, so that the baby will produce their own IgM after having been infected.17,19
Since it has been known that SARS-CoV-2 binds to ACE2 receptors, and there is an increased ACE2 in pregnant females, with a limited number on the placenta, vertical infection through the placenta still may occur.20,21 Until now, there are still researchers who doubt that SARS-CoV-2 can be transmitted vertically; instead they propose that the reactive IgM findings in newborns might be caused by a damaged placenta, which allows the mother’s IgM to be transmitted to the foetus.22
Damages occurring to the placenta could also be explained by the infection of SARS-CoV-2, as a variety of histopathological changes were observed in COVID-19 positive pregnancies, such as fibrin and thrombus formation, avascular villi, and other decidual arteriopathies. The findings of placental pathology cause malperfusion observed from both the maternal and foetal side and could be associated with the worse maternal and foetal outcomes in pregnancy with COVID-19.23-26 One case report conducted by Hosier et al.27 described that high viral load of SARS-CoV-2 RNA resides mostly in the syncytiotrophloblast cells of the placenta. The abundance of SARS-CoV-2 in the syncytiotrophloblast cells might be explained by how ACE2 genes are expressed in those cells at 6–14 weeks of gestation, including in the perivascular cells in decidua, and villous cytotrophoblast.28
It is suspected that there may be a possibility of vertical transmission due to studies showing the discovery of ACE2 receptors even in small amounts in the placenta, which allows vertical transmission through the placenta.21 When there is a bond between SARS-CoV-2 and the ACE2 receptor, transmembrane protease serine enzyme 2 will be activated and facilitate the virus to pass through cells.29 This enzyme is known to be expressed in villous cytotrophoblasts, epithelial glandular cells, and also expressed minimally in syncytiotrophoblast cells.28 This makes it possible to find the SARS-CoV-2 virus RNA in the placenta or amniotic membranes as reported by Penfield et al.30
This study showed similar results with one case report from Wuhan of a neonate born from a female who was IgG anti-SARS-CoV-2 positive, who had normal IgM levels but strong positive IgG results.31
Another study conducted on six neonates from the same region showed strong positive IgG, but all had negative IgM, except for one. Out of the six newborns, one of them had a positive IgG value up to 150 days, and two stayed positive up until the 180th day. The mothers of the two infants also still showed positive IgG on the 180th day, indicating that maternal IgG levels could possibly provide protective IgG against COVID-19 for their infants.32 The amount of IgG antibody titre found in the infants depends on the amount of IgG titre of the mother. Pregnant females who have been infected with SARS-CoV-2 for more than 2 weeks will provide a more adequate antibody titre in the infant.33 Even so, the persistent IgG values on the infants still could be affected by other external factors such as breastfeeding. Breastmilk has been proven to carry anti-SARS-CoV-2 IgG antibodies, whether the antibodies are created from previous COVID-19 vaccination or infection. The IgG antibody is also evident from infants’ stool.34 It has also already been proven that vaccination of mothers against SARS-CoV-2 during pregnancy results to antispike IgG being formed in their infants for up to 3 months.35 Regardless, positive IgG findings in infants are known to help neutralise SARS-CoV-2.36
The presence of IgG against SARS-CoV-2 in newborns who are negative on RT-PCR examination and born to mothers with COVID-19 indicates the possibility of transmission of antibodies from mother to child.37 Maternal immunity can cross the blood–placenta barrier, which can cause the formation of passive immunity in the foetus.38 Therefore, from this study it can be assessed that the relative risk of transmitting COVID-19 antibodies from mother to foetus is 3 times (risk ratio: 3.00; 95% confidence interval [CI]: 1.56–64.26). In contrast to IgM, IgG can be passively transferred from mother to foetus across the blood–placental barrier. Transfer of IgG usually begins at the end of the second trimester and reaches high levels at birth.17,18 Even though the presence of IgG in the infant’s body indicates that the baby has passive immunity, the duration of passive immunity from the mother’s IgG is still unclear.18 There are studies that state that this protective effect of passive immunity does not last long and will disappear after 2 months.33
The possibility of negative IgG findings in the other newborns of this study could be explained due to low maternal IgG titres, or the deliveries happening before IgG transfer to the foetus could occur.39 It should also be put into consideration that serum IgM and IgG can be detected within days up to 1–3 weeks after COVID-19 infection, and IgM antibodies decay earlier than IgG, hence the timing from maternal COVID-19 infection to taking the blood serum sample might affect the subsequent findings.40,41
From the results of this study, there were 165 pregnant females from January–April 2021. Out of 165 pregnant females there were 15 cases positive for COVID-19. From these data, one baby was found with reactive examination results so that the prevalence ratio (PR) could be calculated by comparing the number of cases in the test group with the control group and the results obtained were 11.7 (PR: 11.7; 95% CI: 1.63–35.57 Table 2).
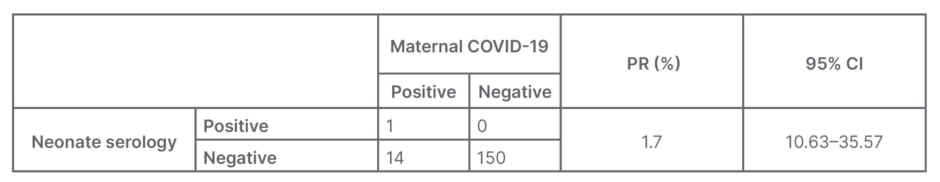
Table 2: Features of vertical transmission in pregnancy with COVID-19 infection.
CI: confidence interval; PR prevalence ratio.
PR results >1 indicate that pregnant females with COVID-19 have a risk of vertically transmitting it to their babies 11.7 times higher compared with those without COVID-19 infection. Nonetheless, until now the evidence of vertical transmission of COVID-19 is still being investigated.
A case study from Iran reported positive SARS-CoV-2 RNA results in the amniotic fluid of a premature baby, who then showed positive nasopharyngeal swab RT-PCR results 24 hours post-partum, but negative RT-PCR results from samples taken from the vaginal secretion and umbilical cord blood.37
Another case study conducted by Kirtsman et al.42 reported the possibility of congenital infection with SARS-CoV-2 as evident by positive RT-PCR swab results from samples taken from the newborn’s nasopharynx, blood (Day 4), stool (Day 7), the placenta, and the mother’s breast milk, along with maternal vaginal swabs. In a larger study of 666 newborns of females positive for SARS-CoV-2, 28 infants (4%) were infected with SARS-CoV-2 after birth.43 Meta-analysis research conducted by Kotlyar et al.44 showed that the combined proportion of vertical transmission of SARS-CoV-2 from 936 neonates born from mothers with COVID-19 was 3.2% (95% CI: 2.2–4.3). Other sites tested for SARS-CoV-2 in this study yielded a variety of positive results, such as 0.0% in amniotic fluid and urine samples, 7.7% in placental samples, 3.6% from the cord blood, 9.7% in anal or rectal swabs, and a seropositivity of 3.7% of neonates. Another study from China showed that the risk of vertical transmission is 16 out of 1,000 live births.45 This shows that the risk of vertical transmission is considerably small.
Some other literature reports that there are no documented cases of intrauterine transmission, and there are cases that only find an increase in the level of COVID-19 IgM in neonates aged 1–2 days. A positive IgM result is not definitive evidence of intrauterine infection because of the possibility of false positives and cross-reactivity that occurs. Maternal immunity can cross the placental blood barrier, which can lead to the formation of passive immunity in the foetus. Moreover, in many cases early infant infection may have occurred due to postnatal contact with infected parents or caregivers.46-49
The method of delivery has also been a topic of concern regarding the risk of vertical transmission. Some studies have found that Caesarean section delivery is more commonly done for females infected with COVID-19.50,51 Vertical transmission may occur through the placenta, through direct contact with maternal blood or vaginal secretion during birth, and through breastfeeding.51 Some may argue that vaginal delivery poses a higher risk of vertical transmission, since there would be a direct contact between the newborn and cervicovaginal secretion, but instead a meta-analysis study from Indonesia, conducted by Sarastry et al.,51 showed that vaginal delivery does not hold a greater risk of vertical transmission of SARS-CoV-2 to newborns compared with Caesarean section deliveries, hence the method of delivery should be based on the obstetric indications. Their findings are in line with the results from the study conducted by Lopian et al.52 and Cai et al.53
Even though vertical transmission is yet to be proven, severity of maternal COVID-19 infection could also be one of the determining factors of vertical transmission happening, since worse COVID-19 severity causes an increase in serum IgM and IgG antibody production against SARS-CoV-2.54
Measures should also be taken to prevent vertical and post-partum transmission of SARS-CoV-2 from mothers to their newborns. One cohort study completed by Tavakoli et al.55 found that using remdesivir for the treatment protocol of pregnant females with COVID-19 is shown to reduce vertical transmission and reduce neonatal intensive care unit admissions. It is also not a contraindication for infants to receive breastmilk from their COVID-19-positive mothers, although its safety and preventive measures against SARS-CoV-2 transmission should be noted, considering that 70% of neonate infections occur through postpartum transmission. Therefore, practising hand hygiene, wearing face masks, and cleaning breast pumps properly before and after breast milk expression is crucial to prevent further transmission.56
One of the limitations of this study was using serum IgM and IgG to describe vertical transmission of SARS-CoV-2 when some studies have shown that anti-SARS-CoV-2 IgG can be transmitted to the foetus post-COVID-19 vaccination; hence, COVID-19 vaccination status should be recorded and considered for future studies.57-59 Moreover, antibody serology testing is less sensitive and specific for COVID-19 detection; therefore, using RT-PCR for diagnosing COVID-19 is preferred and is considered to be more representative for vertical transmission.60 The number of COVID-19 in pregnant cases presented in this study is still very limited, so results of this study should be interpreted according to context. Further research with a longer study design might solve the problem of the amount number of samples included. Certain maternal and neonate variables, such as severity of COVID-19 and gestational age, were also not taken into consideration. The sample characteristics should be considered in future studies to anticipate their effects on the study outcomes.
CONCLUSION
To sum up the findings of this study, the incidence of pregnant females with COVID-19 at the Central General Hospital Prof. Dr. I.G.N.G. Ngoerah Denpasar from January–April 2021 was 15 cases out of 165 caesarean section deliveries, with a prevalence rate of 9.09%. According to this study, COVID-19 infection in pregnancy can increase the risk of vertical transmission of COVID-19 by 11.7 times compared with pregnancy without COVID-19 infection. Further studies considering the duration of study design, amount of sample included, as well as other variables such as vaccination history, COVID-19 severity, and gestational age should be taken into consideration to create a more reliable study.







