Abstract
Introduction: Poor ovarian responders are the most challenging patients in reproductive medicine and no successful treatment has been proposed. Androgens are thought to play an important role during early folliculogenesis and diminished levels are associated with decreased ovarian sensitivity to follicle-stimulating hormone. This study aimed to determine whether pretreatment with testosterone improves the results in poor responders undergoing in vitro fertilisation (IVF).
Materials and methods: This observational pilot study enrolled 33 poor responders undergoing IVF. Eleven patients were pretreated with 250 mg intramuscular testosterone and compared to a control group of 22 patients. The participants were tested for free testosterone, dehydroepiandrosterone sulfate, sex hormone binding globulin, and anti-mullerian hormone (AMH).
Results: The two groups had similar baseline characteristics. Significant improvement was reached in the hormones free testosterone, dehydroepiandrosterone sulfate, and sex hormone binding globulin in the testosterone-pretreatment group. No difference was detected in antral follicle count (5.06 versus 4.24); AMH (0.51 versus 0.53), mature oocytes (2.2 versus 2.32), and the number of embryos (1.2 versus 1.33) between the study and control groups, respectively. There was a slow improvement in fertilisation rate but without any significance (62.97% versus 57.61%). However, the cancellation rate of the ovarian stimulation was much greater in the control group (18.18%) in comparison with the study group (0.0%). Pregnancy rate (PR) in the testosterone group was higher than controls (PR per cycle: 27.3% versus 4.6; p=0.09).
Conclusion: Based on the limited number of patients studied, pretreatment with testosterone seems to improve PR and cancellation rate in poor responders but failed to affect antral follicle count, AMH, and the number of mature oocytes and embryos. Given these results, further research would provide more certainty.
INTRODUCTION
The most challenging patients for fertility care providers are the poor ovarian responders (POR). During the years, many attempts have been made to define the profile of POR but the most useful classifications remain the Bologna criteria 2011 and the later Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) criteria.1-5 In 2016, POSEIDON6 were developed, which are a more specific and more flexible prognosis concept for improving the management of patients undergoing assisted reproductive technologies. The proposed POSEIDON stratifies patients into four groups on the basis of age (over and under 35), anti-mullerian hormone (AMH) levels, and antral follicle count (AFC); Group 1 and 2 are defined as suboptimal responders with good prognosis and Group 3 and 4 as low responders with poor prognosis. This classification was created to serve as a guide to personalise treatment protocols and improve the management of low-prognosis patient to maximise in vitro fertilisation (IVF) success.
Reproductive plans are often delayed and the proportion of patients with POR has become massive. Different pharmacological approaches have been proposed to improve the outcomes of IVF treatment but none have been established with certainty. In recent decades, there has been an increased interest in the role of androgen supplementation while undergoing IVF. In 2000, Casson et al. first suggested that patients with POR could benefit from androgens.7 Thereafter, a series of studies of patients with POR reported that dehydroepiandrosterone (DHEA) supplementation not only improves oocyte yield but also positively affects the egg and embryo quality and IVF pregnancy rate (PR).8-17
However, recent large-scale randomised trials have failed to confirm this, and highlight free testosterone (T) levels as a more appropriate androgen in the improvement of the ovarian reserve.18,19 It has been established that there is a direct T effect on the ovaries through androgen receptors (AR), and sufficient AR are necessary for normal follicular development and function.17,18
Granulosa cells (GC) express AR at early stages of folliculogenesis, with these stages of follicle maturation occurring months before ovulation. Given this, it would be logical to assume that longer androgen supplementation would be associated with better results for follicular activation and recruitment, and thus reach a larger pool of gonadotrophin-sensitive follicles and respectively more oocytes, which appear to improve reproductive outcomes.19,20
Certain modalities have been tested through different studies and trials. In daily practice, the most commonly used T agents, before or during ovarian stimulation, are transdermal testosterone. Some meta-analyses evaluated the effect of different doses or duration of treatment, but the most common duration of therapy was 21 days.19,20 The physiological cycle of the transition of primordial to preantral follicles lasts for 290 days, but in this process the levels of expressed AR are low. However in the preantral stage of follicular development, androgens, through a synergistic interaction between nuclear and extranuclear signalling, induce the expression of AR in GC, contributing to follicular survival and growth. Simultaneously, this increases follicle-stimulating hormone (FSH) receptors (FSHR) that enhance the sensitivity of preantral follicles toward FSH actions. These androgen–AR actions together promote preantral follicle growth and transition to antral stage, taking at least 65 days (Figure 1).21,22
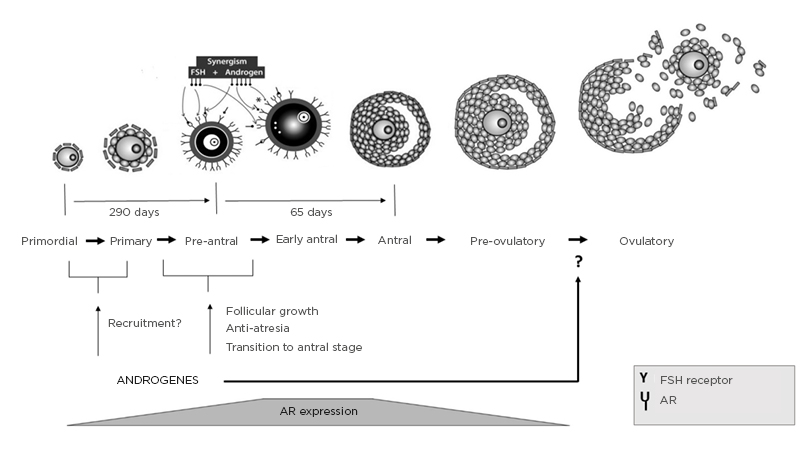
Figure 1: Folliculogenesis and physiological actions of androgens in follicular development, with synergism between androgens, AR, and FSH in preantral follicle activation and maturation.
AR: Androgen receptor; FSH: follicle-stimulating hormone; FSHR: follicle-stimulating hormone receptor.
Adapted from Gleicher N et al.21
Beyond the above-mentioned data, this study aimed to investigate the effects of testosterone pretreatment on the ovarian reserve and IVF outcome in patients with POR but with longer exposition and higher dosage of testosterone administered intramuscularly.
MATERIALS AND METHODS
An observational pilot study was performed at the IVF department of a private obstetrics and gynaecology hospital. The inclusion criteria were female patients with POR who had at least one previous failed or cancelled IVF cycle and met criteria for POSEIDON Group 4.5,6 The patients in the study had a mean age of 39.4 years, mean AMH levels of 0.52 ng/mL, AFC <5, number of retrieved oocytes <5, and level of oestradiol on the day of human chorionic gonadotropin therapy <1,200 pg/mL. No significant difference was observed in demographic and baseline characteristics between the two groups. Exclusion criteria were uterine malformations, hydrosalpinx, FSH >20 IU/L, or severe male factor.
Pretreatment with Testosterone in the Study Group
In all eligible patients, following signed informed consent, 250 mg of intramuscular testosterone was administered twice for 6 weeks. The active substances in the injections were testosterone propionate 30 mg, testosterone phenylpropionate 60 mg, testosterone isocaproate 60 mg, and testosterone decanoate 100 mg.
Ovarian Stimulation Protocol
IVF embryo transfer procedure was performed in all patients. A gonadotropin-releasing hormone antagonist protocol was used for the ovarian stimulation. After confirming baseline blood levels and excluding any functional ovarian cysts, ovarian stimulation with follitropin alfa was started on Day 2 of the menstrual cycle and 4 weeks after the last testosterone application. Patients in the control group underwent the same protocol, without receiving testosterone pretreatment. During ovarian stimulation, regular transvaginal ultrasound scanning and monitoring of serum concentrations of luteinising hormone, oestradiol, and progesterone were undertaken. Ovum pick-up was performed 34–36 hours after the subcutaneous administration of 5,000–6,500 IU human chorionic gonadotropin for the ovulation trigger, and the matured oocytes were fertilised by an intracytoplasmic sperm injection. All embryos were transferred at the cleavage or blastocyst stage. Luteal support was performed via transvaginal administration of progesterone, starting on the day of ovum pick-up.
Hormonal Measurements
All patients were tested for T (nmol/L), DHEA sulphate (DHEA-S) (µmol/L), sex hormone binding globulin (SHBG) (nmol/L), and AMH (ng/mL). Patients eligible for T treatment were those with SHBG <80 nmol/L, T less than one-third of the normal range, and DHEA-S within the normal range. All hormones were analysed before and after T treatment in the tested group.
Outcome Measures
The primary outcome measure was the PR. The secondary outcome measures included the number of antral follicles, developing embryos, and mature (M2) oocytes (COC); fertilisation rate (FR); and cancellation rate (CR). Additionally, changes in the serum hormonal levels of AMH, T, SHBG, and DHEA-S were observed.
FR was calculated by dividing the number of fertilised oocytes by the number of M2 oocytes. Embryo quality was assessed according to morphological criteria based on the assessment of the blastomeres and the degree of blastomere fragmentation. Clinical pregnancy was defined as the presence of an intrauterine sac with fetal heart palpitation.
Sample Size
The above-described criteria were observed for the selection of patients who were able to receive testosterone therapy. From all tested patients, 33 met eligibility criteria and, after obtaining their informed consent, 11 patients were enrolled in the T pretreatment group and 22 in the control group.
Statistical Analysis
Statistical analysis was performed. The odds ratio, with its standard error and 95% confidence interval, were calculated. For test of significance, the p value was calculated and statistical significance was considered at p<0.05. All analyses were performed with MedCalc® statistical software.
RESULTS
All 33 patients were divided into two groups: the study group of 11 participants received pretreatment with 250 mg intramuscular testosterone twice, every 3rd week and the other 22 participants formed the control group without pretreatment.
The main demographic and baseline characteristics of all patients were very similar, including age, BMI, causes and duration of infertility, AFC, DHEA-S, T, SHBG, and AMH, with a mean BMI of 25.6 and mean age of 39.6 years. A significant improvement was reached in the levels of T, DHEA-S, and SHBG in the T pretreatment group (Table 1). No differences were detected in the AFC between the groups (5.06 versus 4.24), AMH (0.51 versus 0.53), number of M2 oocytes retrieved (2.20 versus 2.32), and the total number of embryos (1.20 versus 1.33) (Table 1). The analysis showed a slow improvement of the FR in the study group but without a significance (62.97% versus 57.61%, respectively; p=0.6). However, the CR of the ovarian stimulation in one cycle was much larger in the control group in comparison with the study group (18.8% versus 0.0%; p<0.05). The results showed an almost eight-fold increase in the odds of pregnancy after T treatment in comparison with no pretreatment; PR per cycle in the interventional group was 27.3% and 4.6% in the control group, with odds ratio 7.88 in 95% confidence interval (p=0.09) (Table 1 and Figure 2).
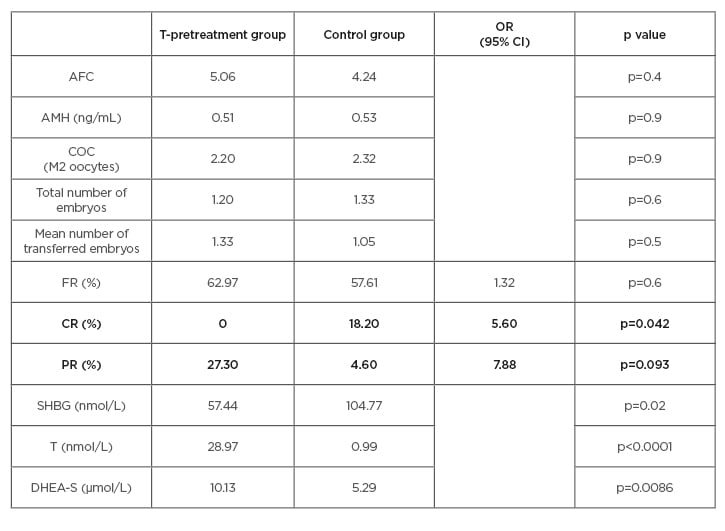
Table 1: The main results in the testosterone-pretreatment group and in the control group.
AFC: antral follicle count; AMH: anti-mullerian hormone; CI: confidence interval; COC: cumulus–oocyte complex; CR: cancellation rate; DHEA-S: dehydroepiandrosterone sulfate; FR: fertilisation rate; OR: odds ratio; PR: pregnancy rate; SHBG: sex hormone binding globulin; T: free testosterone.
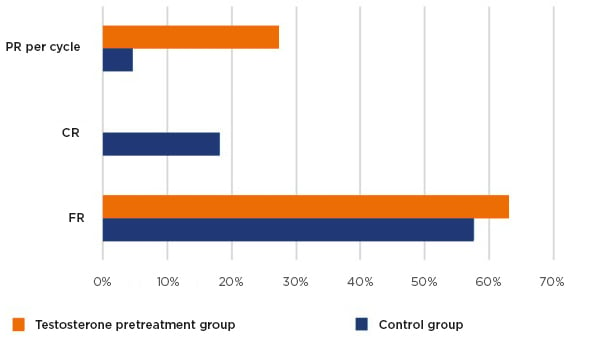
Figure 2: Pregnancy rate, cancellation rate, and fertilisation rate results.
CR: cancellation rate; FR: fertilisation rate; PR: pregnancy rate.
DISCUSSION
There is evidence that testosterone levels in individuals with diminished ovarian reserve decrease significantly, irrespective of age or premature ovarian ageing.23,24
According to the ‘Two cell, two gonadotropin’ theory of ovarian steroidogenesis, oestrogen’s synthesis in GC originates from androgens from thecal cells. Over the years, understanding of the effect of androgens on the ovary process and female fertility has developed significantly. Androgens are considered to be detrimental to ovarian function, and sufficient androgen actions through the AR are necessary for normal follicle development and function.21,25 AR expression can be found in GC of mostly immature preantral and early-antral follicles; thereafter, they decrease with advancing follicle maturation, suggesting the importance of androgens mainly in the early stages of follicle maturation21,22,26 (Figure 1). Many studies demonstrate androgens as essential for follicular recruitment, follicular growth, and reduction in GC apoptosis leading to an increase in the number of growing follicles.27-29 Additionally, AR actions induce the expression of the micro-RNA miR-125b that decreases pro-apoptotic proteins. It has been proposed that in the ovary androgens maintain a certain level of miR-125b expression, essential for the balance between follicular survival and atresia.30
Furthermore, androgens have been found to induce FSHR mRNA expression during preantral to antral follicle progression, whether this induction by androgens is mediated through androgen–AR response or by direct synergism between androgens and FSH in the ovary. This suggests that androgen stimulation enhances follicular sensitivity toward FSH actions by increasing FSHR levels, which potentially contributes to follicle growth (Figure 1).31-36
It is known that androgens, acting via the AR, may also regulate the expression and action of key ovarian growth factors during different stages of follicle growth, which indicates that an intraovarian growth factor system plays an essential role in ovarian follicular development, regulated by androgen–AR actions.37 A study demonstrated that during the ovulation process the androgen–AR pathway also plays a role in the last stage of folliculogenesis. Androgens produced by the luteinising hormone surge are likely to act through the AR and may be involved in the ovulatory process by directly regulating the expression of COX2 and AREG genes and their actions (Figure 1).38
Taking into consideration the above-mentioned evidence, long pretreatment with testosterone could be beneficial for ovarian response. Despite the significantly higher testosterone level after 6 weeks of administration compared with no pretreatment, no statistically significant changes were detected in this study in the mean number of AFC (5.06 versus 4.24, respectively), mature oocytes retrieved (2.2 versus 2.32), and total number of embryos (1.2 versus 1.33) (Table 1). In agreement with this study, Massin et al.39 performed a randomised controlled trial with 49 patients and found a nonsignificant increase with testosterone pretreatment (10 mg/day for 15–21 days) on the number of COC. Six years later, a systematic review and meta-analysis was performed by Gonzalez-Comadran et al.40 and no differences were observed regarding the number and quality of the oocytes retrieved. In 2016, 26 patients were randomly pretreated with T, but it failed to increase the COC.41 Contrary to this, Noventa et al.42 observed that a higher number of total oocytes, M2 oocytes, and total embryos were developed after T therapy. The same was shown by Kim et al.,43,44 in a significant improvement in the number of COC retrieved, and showing that the level of success is time dependent (COC retrieved with pretreatment for 2 weeks: 4.3 versus 1.6; for 3 weeks: 5.3 versus 2.0; and for 4 weeks: 5.8 versus 1.9). These inconsistent results highlight the possibility that the evaluated studies might differ by the type of substance, the timing and the duration of the treatment, and by the mechanism of action.
Apart from the idea that androgens may regulate AMH and that some studies show a possible positive relationship between androgens and AMH levels in follicular fluid, the direct evidence of androgen-induced AMH expression and its underlying mechanism in GC are still lacking.45 The same result was observed in this study where no difference in serum AMH was observed after T therapy (0.51 versus 0.53). Vuong LN et al.46performed long-term intraovarian androgen priming but also did not find any significant effect on AMH level. However, despite androgens improving recruitment and activation of prenatal follicles and the fact that AMH is synthesised in GC, there are insufficient studies observing the effect of testosterone on AMH.
This study revealed a significant improvement in the CR of ovarian stimulation in the T group (0.0% versus 18.2% in the control group; p<0.05). This is important in impacting time, money, and patients’ hope. This effect of testosterone could be explained as a result of the increased levels of FSHR mRNA in GC after androgen supplementation and supports the fact that androgens enhance follicle responsiveness to FSH, particularly in early antral stages, and the theory of a synergistic effect of androgens with FSH over folliculogenesis.32,34
Despite these conflicting results, the vast majority of studies claimed greater clinical PR after testosterone therapy, consistent with this study’s results (PR per cycle: 27.3% in T group versus 4.6% in control group; p=0.09, close to significance). Noventa et al.42 performed a meta-analysis of available randomised controlled trials on the effect of transdermal testosterone, demonstrating a higher clinical PR and live birth rate. Recently, Vishwakarma et al.47 reported an improvement in the numbers of cryopreserved embryos per cycle and hence the cumulative PR.
Limitation and Strengths of the Study
This observational pilot study has some potential limitations that need to be considered; mainly, the relatively small number of patients included. This low rate of events decreases confidence in the results. Secondly, there are no other trials with intramuscular application of testosterone, so comparison was made with other types of administration which could give some bias. However, this limitation simultaneously could be counted as a strength of the study, because it is the first report of this type. In the literature, the most common route of administration was transdermal and only one other study reported an alternative method: intraovarian priming.46
CONCLUSION
Long-term testosterone pretreatment could be considered promising in IVF treatment of patients with POR. The findings of this study show improvement of the PR and CR. No effect was observed on AFC, AMH, M2 oocytes retrieved, and the total number of embryos. Due to the limitations described above, more studies should be performed with a larger population and better adjustment with ovarian physiology by dose, timing, type, and duration of the testosterone therapy.







