Abstract
In vitro fertilisation (IVF) is a complex procedure, the success of which is dependent on several factors at every step of the process. Despite major advances, successful implantation rates in IVF remain low. Aside from the status of the embryo and endometrium, embryo transfer (ET) plays a major role in implantation. There are numerous variables in ET that are causative factors for IVF success. In this article, the authors discuss whether the stage at which (cleavage versus blastocyst) ET occurs; a fresh or frozen ET; and the technique of ET affects the results of an assisted reproductive technology cycle. Blastocysts had higher implantation potential than cleavage-stage embryos and it was also observed that extended embryo culture was not related to increased adverse obstetric and perinatal outcome. Though freezing has several advantages over fresh cycles, one must remember that evidence is still lacking for its use in all patients. Elective cryopreservation of all embryos with transfer in subsequent frozen ET cycles may be requited in cases at risk of developing ovarian hyperstimulation syndrome, women undergoing preimplantation genetic screening or preimplantation genetic diagnosis for genetic analysis, polycystic ovarian syndrome patients, and those who have high progesterone levels on the day of human chorionic gonadotropin, but to date it is debatable whether a freeze-all strategy will benefit normal and poor responders. For an optimal ET technique, the use of soft catheters and performing the process under ultrasound guidance will improve results by making it less traumatic, standardised across centres, and more technically precise.
INTRODUCTION
Despite major advances, in vitro fertilisation (IVF) implantation rates (IR) remain low and only a small percentage of patients achieve pregnancy. There are various factors that affect results. Aside from the health of the embryo and endometrium, embryo transfer (ET) plays major role in implantation. It is also important to understand that the variables in IVF that can affect success of the process are numerous; therefore, it is very difficult to pinpoint one factor of ET to study regarding outcomes.
There are numerous variables in ET that are causative factors for IVF success, such as the transfer of cleavage stage versus blastocyst embryo, fresh versus frozen, and the technique of ET. In this article, the authors discuss these factors and study their impact on the success of IVF.
CLEAVAGE VERSUS BLASTOCYST EMBRYO TRANSFER
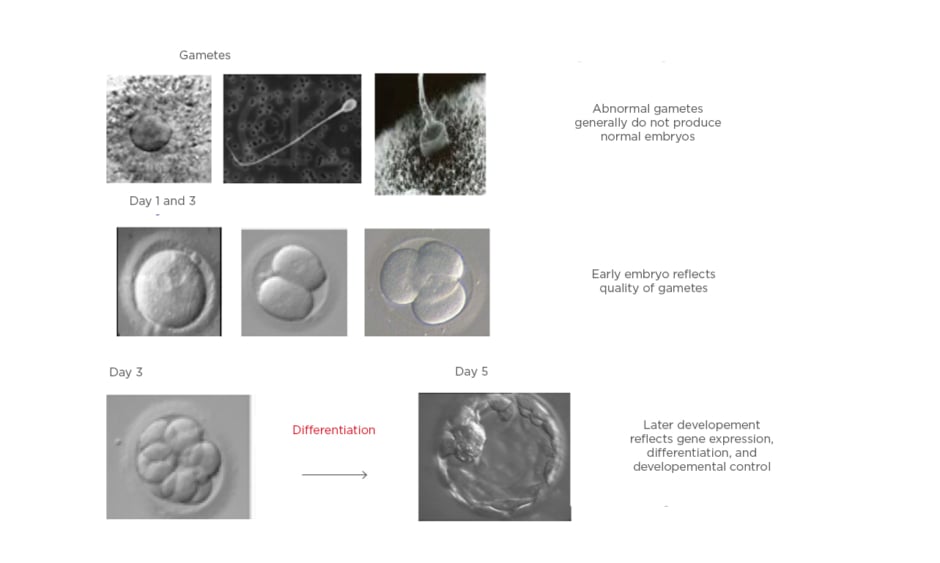
Over the past two decades, there has been an increased interest in blastocyst transfers because it has a better implantation rate than a cleavage stage embryo. To date, most clinics still conduct cleavage stage ET because it is a standard global practice, and a low developmental rate of embryos past cleavage stage is seen if the embryology lab is not equipped for a blastocyst culture. The main advantage of blastocyst transfer is improved embryo–endometrium synchrony, and therefore higher chances of implantation, because the process of blastocyst implantation more closely mimics natural conception.1,2 The other advantage of blastocyst transfer is improved embryo selection, with a significant increase in the live birth rate per started treatment compared with cleavage stage ET; however, blastocyst transfer requires optimal laboratory conditions.1,2 Improved laboratory standards and better culture media have made extending culture to blastocyst stage a reality to identify the embryos with maximum implantation potential. Figure 1 outlines the process of transferring the embryos at the blastocyst stage.
Advantages of blastocyst stage ET over earlier stage ET are as follows:
- Premature exposure of an early stage embryos to uterine environment may cause homeostatic stress, resulting in reduced implantation potential.
- More physiologically beneficial because there is synchronisation of the embryos with the uterine endometrium.
- Embryonic block at the 8-cell-stage has been overcome; thus, improved embryo selection can be conducted, allowing best embryo to be transferred.
- The extra time in vitro allows for the selection of the embryos with a high implantation potential.
- IR of Day 5 embryo (single blastocyst transfer) is 49%, compared to 33% for Day 2/3 embryos (single ET [SET]) in patients ≤36 years, thus better pregnancy rates (PR) are observed with SET if blastocysts are transferred.
- Selection of a good quality embryo allows for SET, decreasing multiple PR.
- Reduced myometrial and endometrial contractions when blastocysts are transferred with a lower risk of being expelled.3
- Allows enough time for preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS) results with a Day 3 biopsy.
- Comprehensive chromosome screening is possible with trophectoderm (TE) biopsy. Increasing evidence from randomised controlled trials that have shown that comprehensive chromosome screening at the blastocyst stage improves IR and PR.4-6
- Fewer embryos are required for higher IR and PR.7
- A higher live-birth rate following fresh transfer in patients with a good prognosis, patients with repeated miscarriages or IVF failures, and transfer of euploid embryos after PGS.
- Better cryopreservation results with vitrification of blastocysts, which increases cumulative PR.
- Significantly lower risk of ectopic pregnancy following transfer of a single frozen blastocyst.8 It was also seen that the ectopic pregnancy rate was lower in frozen thaw cycles compared to fresh cycles; 1.9% for Day 3 frozen thaw cycles, compared to 0.3 % for Day 5, and 0.5 % for Day 6.9
- Because of the larger diameter of blastocysts, the rate of ectopic pregnancy was decreased after blastocyst transfer.7 The ectopic pregnancy rates were 2.1–2.4% for fresh Day 3 transfers, compared to 1.6–1.7% for fresh Day 5 transfers.9-10
- Ectopic pregnancy rate was also related to the day the embryos were frozen. This study found a significantly lower risk of ectopic pregnancy after frozen embryo transfer if the embryos were vitrified on Day 6 (0.6%) as compared to Day 3 (3.1%) or Day 5 (2%).11
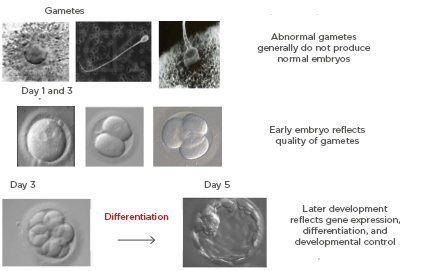
Figure 1: Blastocyst formation as a result of differentiation.
One study showed that extended in vitro culture was not associated with increased adverse obstetric and perinatal outcome in pregnancies resulting from fresh SET.12 Nonetheless, the effects of prolonging embryo culture to Day 6 must be considered. Several researchers have shown lower IR with Day 6 embryos compared with Day 5 blastocysts (36.34% versus 19.00%13 and 22.10% versus 3.60%,14 respectively). When fresh ET were compared with frozen ET, the difference in the implantation potential of Day 5 versus Day 6 blastocysts occur as a result of advanced endometrial development in controlled ovarian stimulation (COS) cycles with fresh ET.13,15 This is because slower embryos are less likely to implant, as the embryos miss the implantation window. Clinical PR were similar between blastocysts cryopreserved on Day 5 and those cryopreserved on Day 6 (32% versus 28%).15 Moreover, the grade of blastocyst will also influence the IR and PR.16 When discussing the grade of blastocyst, one should consider expansion and hatching first among the three morphological parameters when selecting a blastocyst for transfer; these two parameters have a high predictive value for live birth. Furthermore, inner cell mass and TE grade must be considered. Transfer of a blastocyst with inner cell mass Grade A may reduce the risk of an early pregnancy loss,17 while the presence of cytoplasmic strings and vacuoles decrease the IR.18-22 Blastocyst-stage ET allows the transfer of fewer embryos of higher quality, thus eliminating the potential risk of higher order multiples while maintaining high rates of pregnancy per transfer.13
Despite the advantages, most clinicians are unsure whether to transfer a cleavage cell embryo or a blastocyst, because there is a fear of losing embryos by culturing them to the blastocyst stage, especially if the Day 3 embryo is not of good quality or when there are few oocytes and embryos. This may result in cancellation of ET, thus resulting in the loss of an IVF cycle. When no blastocysts form, this could be the result of poor developmental potential or because of poor in vitro culture conditions; furthermore, the impact of prolonged in vitro culture is not clear. Additionally, fewer embryos are cryopreserved with blastocyst transfers (odds ratio: 0.48; 95% confidence interval: 0.40–0.57 [14 studies, 2,292 women]).1,23 Moreover, though blastocyst transfer improves the odds of transferring a viable embryo,24 the prolonged in vitro culture does not guarantee euploidy. Recent studies have shown that chromosomally abnormal embryos can become blastocysts.25
Lastly, a cost–benefit analysis of the two procedures should also be conducted. There is always a higher cost related to blastocyst culture and transfer due to the requirement of additional incubators, culture media, more laboratory staff members, expertise in blastocyst quality assessment, and cryopreservation protocols.
Upon considering the advantages of blastocyst versus cleavage cell ET, although the live birth rate is higher in blastocyst stage transfer, the balance tilts more in favour of cleavage-stage transfer. There was also an increased chance of monozygotic twinning (MZT) with blastocyst transfer,26,27 high risk of sex ratio imbalance to male (p=0.01),27 increased risk of preterm birth,28-31 and large-for-gestational-age babies compared with cleavage cell ET offspring.30,32 Dar et al.29 reported a significantly higher incidence of congenital anomalies for babies born after blastocyst stage transfer. No difference in maternal outcome for pre-eclampsia, placental abruption, placenta previa, postpartum haemorrhage, premature rupture of membranes, and gestational diabetes in both the blastocyst group and the cleavage stage ET group was observed. Genetic and epigenetic changes as a result of extended in vitro culture are hypothesised to be the one of the drivers of the adverse perinatal outcomes associated with blastocyst stage ET.
The cost of multiple pregnancy must be added to the cost of extended culture, because blastocyst transfer has a higher risk of MZT.33 The incidence of MZT is much higher in women aged <35 years undergoing blastocyst transfer compared with those aged >35 years (3.4% versus 2.1%; p=0.01).34 Patients aged >35 years showed no difference in MZT rates when comparing stages of transfer. When analysing embryologic parameters, those with ≥4 6–8-cell embryos, and those with >75% of all embryos with 6–8-cells were more likely to have MZT if blastocyst transfer was to occur. MZT rate resulting from an elective SET from a high-quality embryo cohort (1.9%) was similar to the overall MZT rate in cleavage-stage embryos (1.9%).
Moreover, there are no studies on long-term outcomes in children born after blastocyst transfer.31 Despite the shortfalls of blastocyst transfer, most clinics performing single blastocyst transfer, aiming to achieve a healthy singleton live-birth and consequently minimising the number of multiple births and their associated complications while still maintaining PR per transfer. The evidence for the above shortcomings of blastocyst transfer is either of low or very low quality must also be taken into account.
Newer technologies should be evaluated for better selection of embryos on Day 3, so as to give maximum pregnancies with fresh and frozen embryos per treatment cycle.
FRESH VERSUS FROZEN EMBRYOS TRANSFER
The conventional indication for freezing embryos in assisted reproductive technology (ART) is the availability of surplus embryos, which increases the cumulative conception rate and decreases the multiple PR by restricting the number of embryos transferred. Freezing is also used for women at risk of ovarian hyperstimulation syndrome (OHSS), those with progesterone elevation on the day of human chorionic gonadotropin (hCG) administration, and to allow personalised ET based on endometrial receptivity profile in women with recurrent implantation failure. Moreover, freezing the embryos also gives additional time for new invasive and non-invasive methods of embryo selection in patients undergoing pre-implantation genetic screening or diagnosis (PGS, PGD). Freezing is also mandatory for women undergoing fertility preservation before cancer treatment.
Fresh ET has been the norm in ART for the last three decades. Over the last 30 years, IVF treatment and research has made major progress in improving stimulation protocols and fertilisation procedures, optimising embryo culture conditions, and preventing premature luteinisation; however, only a marginal improvement has been seen in the IR and PR. It is understood that 30% of embryos are lost at the pre-implantation stage, 30% are lost after embryo implantantation but prior to the missed period and detected only by positive beta hCG, and 10% are lost after the missed period.35 Thus, disturbance in embryo–maternal dialogue is the major reason for pregnancies to terminate at end of the peri-implantation period.
The elevated oestrogen levels due to multi-folliculogenesis seen with controlled ovarian stimulation cycles have a negative impact on endometrial angiogenesis, implantation, gene expression, and factors responsible for endometrial receptivity.36-38 Thus, modification of the uterine environment as a result of COS may affect the IR in a fresh ET cycle as compared to a frozen ET (FET) cycle, in which the deleterious effects of COS on the endometrium can be avoided with a better outcome.39-41 There was only one publication by Shih et al.42 that highlighted the physical effects of freezing and thawing embryos may filter out embryos of borderline quality with lower implantation potential, thus resulting in better IR and PR after FET.
There has been a statistically significant increase in the CPR and higher ongoing pregnancy rate (OPR) after an elective FET compared with fresh ET with the use of vitrification techniques for embryo cryopreservation, which may reduce embryo cryodamage and therefore increase success rates.43 FET also eliminates the effect of COS on endometrial receptivity. The publication by Shapiro et al.39 demonstrated a higher PR and OPR with cryopreserved embryos. The fallacy with this study was that the fresh ET included both Day 5 and Day 6 fresh blastocysts; the lower OPR reported with fresh blastocysts transfer could be related to the Day 6 transfers, which may result in embryo–endometrial asynchrony.
Chen et al.44 demonstrated a statistically significant 7.3% absolute increase in live births following delayed, FET in women with polycystic ovary syndrome (PCOS). This paper reported an overall low live birth rate in the fresh ET group due to an increase in pregnancy loss, both biochemical and clinical pregnancies, along with second trimester abortions. The increased risk of miscarriages in the fresh ET group could be a result of abnormal placentation and abnormal hormonal milieu, which can affect the endometrium. Maternal response to pregnancy is affected by the metabolic effects of PCOS. These results may not extrapolate to include women with <15 oocytes. Shapiro et al.39 demonstrated a higher CPR and OPR even in normal-responders in FET cycles as compared to fresh ET cycles. Blockeel et al.45 looked at the SWOT (strengths, weaknesses, opportunities, and threats) analysis of a freeze-all strategy. Though freeze-all has the advantages of decreased risk of OHSS and better endometrial receptivity with improved PR, the clinical benefits of a freeze-all strategy should be looked at through well-designed clinical trials prior to shifting our current ART practice.45 Roque et al.46 suggested that a freeze-all strategy is not suited for all IVF patients. Freeze-all is indicated in women with risk of OHSS development and in patients with supra-physiologic hormonal levels during the follicular phase of COS; these include high oestradiol levels and elevated progesterone levels on the day of hCG. Therefore, before advocating the freeze-all approach, an evaluation of the pros and cons, including potential costs and delays in treatment, and potential risks associated with this strategy should be conducted. As most studies were carried out in women with normal or high ovarian response,39,44,46-48 the same cannot be extrapolated in women who are poor ovarian responders.
A recent meta-analysis by Maheshwari et al.49 showed that the relative risks of small for gestational age, preterm birth, low birth weight, perinatal mortality, and antepartum haemorrhage were much lower in women carrying singleton pregnancies following IVF and who underwent FET rather than fresh ET. There were several confounding factors which were not taken into consideration in the studies included. These confounding factors were age, smoker status, parity duration of infertility, pre-existing medical illness, and method and stage of cryopreservation of embryos.49
There was one systematic review and meta-analysis that investigated the effect of cryopreservation on the perinatal outcome after cleavage stage and blastocyst stage ET and found no difference in the rate of very preterm birth, low birth weight, very low birth weight, and congenital anomalies between the two groups, irrespective of the cryopreservation procedure. This meta-analysis reported a higher incidence of large for gestational age babies in the blastocyst group as compared to cleavage stage transfers after FET.50 FET cycles have the clinical advantages of flexibility of ovum pick up scheduling, less stringent cycle monitoring, and obtaining more oocytes with reduced risk of OHSS, but it also gives a false sense of security and over stimulation can be dangerous.51,52
When considering freeze-all and elective FET for all approaches, the cost-effectiveness and safety, as well as the emotional status of the patient, must be evaluated; there are very few studies that have examined these factors. One publication by Roque et al.53 reported the cost-effectiveness of FET and fresh ET, but all IVF specialists are aware that cryopreservation of all embryos and subsequent FET incurs more cost compared to fresh ET. The costs associated with freeze-all include the cost of freezing embryos and the cost of the FET procedure, which is 30% higher. Moreover, the time to pregnancy also increases considerably, potentially resulting in epigenetic changes in the embryo.
With regard to the safety issue associated with the technique, there is evidence suggesting a higher incidence of large for gestational age babies54,55 and a higher risk of placenta accreta56,57 after FET. It is still controversial whether the risk of hypertensive disorders is also increased in women undergoing FET, as compared to those who undergo fresh ET cycles. There are publications that have reported a higher risk of pregnancy induced hypertension.58 There could also be an increased psychological burden due to postponement of ET and increase of time to pregnancy.52,59 Apart from the perinatal and maternal issues, the cryopreservation procedure itself can have negative effects (Figure 2), including cryodamage, toxicity of cryoprotectants, potential equipment failure or LN2 supply failure, breach of packaging and contamination in storage, and loss of embryos.52
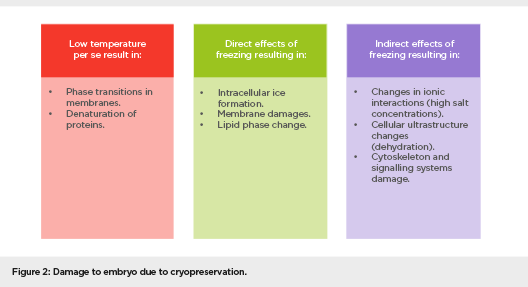
Figure 2: Damage to embryo due to cryopreservation
Cryopreservation may also have an effect on the integrity of the genome, and long-term follow-up of the children born after FET is therefore necessary. Cryopreservation can influence genome integrity by a number of mechanisms, including increasing reactive oxygen species, the use of cryoprotectants, exposure to low temperature, the incorporation of calcium and zinc molecule in DNA–protamine complex, and intracellular crystallisation.60
Results of cryopreservation may also vary due to variables such as embryo quality and morphology at freezing and post thaw and cryopreservation procedure, which can impair pre-implantation development. This can result in reduced cell numbers at the blastocyst stage and affect the survival rates of embryos after cryopreservation.61,62
Thus, the available evidence does not justify a change in practice at present from fresh ET to freeze for all. But improved success rate of IVF by electively freezing all embryos with routine use of frozen and thawed ET has been a matter of debate.63 There is a need for large multicentre, randomised controlled trials to evaluate the clinical and cost-effectiveness, as well as acceptability of elective FET versus fresh ET.
OPTIMISATION THE EMBRYO TRANSFER TECHNIQUE
ET is the least successful step in the IVF process and, apart from embryo quality and endometrial receptivity, it is an important factor determining the outcome. Approximately 30% of ART failures are due to the poor performance of ET.64-67 The procedure for ET is described in Figure 3.
Figure 3: Steps of transfer technique
Factors that may Affect the Success of Embryo Transfer
- Ease of procedure.
- ET catheter type: soft versus hard ET catheter.
- Removal of cervical mucus.
- Use of ultrasound guidance.
- Position of embryo deposition in the uterus.
- Position of the air-medium content in the catheter and the amount of media transferred.
- Duration of ET.
- Presence or absence of the blood on the catheter tip.
- Retention of embryos in the catheter.
- Excessive uterine contractions after ET.
- Microbiological factors in the cervix and bacterial contamination of the catheter.
- Rest after ET.
- Experience of the physician.
Key elements for successful ET are an easy, atraumatic transfer, which is standardised and technically precise, without blood or mucus, and this can be achieved by performing a trial transfer using a soft catheter and performing the procedure under ultrasound guidance. Optimal placement of the embryos, 1.5 cm from the fundus, by injecting them slowly as confirmed by ultrasound, increases the PR. Negotiation of a difficult or stenotic cervix, identified earlier by pre-cycle dilatation, can be achieved using a malleable stylet at ET that is guided by ultrasound. One also needs to minimise embryo stress by minimising transfer time and maintain the temperature and pH of the culture media.
Implantation can be optimised by minimising contractions, avoiding trauma to the cervix or fundus, and performing a blastocyst transfer instead of a cleavage cell transfer.68
Despite all these precautions, ET can still prove to be difficult. Difficult transfer can arise as a result of anatomical distortion of the cervix by previous surgery or fibroids or due to congenital anomaly, presence of pronounced uterine flexion, presence of scarring in the lower uterine segment, or presence of a distorted endometrial cavity. One can overcome a difficult ET by performing a mock transfer, using stiffer and more rigid catheter systems, gently maneuvering the vaginal speculum until resistance at the internal ostium is felt.69,70 After which, moderate cervical traction to straighten the utero-cervical angle, using a malleable obturator, followed by an inner catheter with embryos, using a co-axial or echo tip catheter system.71 The use of ultrasound guidance to facilitate ET, and using trans-myometrial (vaginal or abdominal) surgical ET or a trans tubal ET is recommended in rare cases.72
CONCLUSION
IVF is a complex procedure, the success of which is dependent on several factors that are involved at every step of the process. Some of the factors that impact the outcome of IVF are gamete and embryo quality, stimulation protocols, endometrial receptivity, and ET. In this article, the authors have discussed whether the time (cleavage versus blastocyst) of ET, the use of fresh or frozen embryos, and the technique of ET affects the results of an ART cycle.
Providers of IVF treatment have an obligation to minimise complications associated with IVF and safeguard the long-term health of future generations. Blastocysts have a higher implantation potential than cleavage-stage embryos, and it has also been observed that extended embryo culture was not related to increased adverse obstetric and perinatal outcome. With this concept in mind, most clinics worldwide have moved to single ET at the blastocyst stage with the aim of achieving a healthy singleton live-birth, minimising the number of multiple births and their associated complications, while still maintaining PR per transfer. While blastocyst transfer permits embryo self-selection, it also exposes the embryos to possible harm, due to culturing in the in vitro environment. Both effectiveness and safety should be weighed to permit evidence-based decisions in clinical practice. For extended culture to the blastocyst stage to be effective, technical refinements in laboratory equipment and processes are required, which may be an expensive adaptation in low-resource settings.
PR associated with frozen-thawed embryos would appear to be comparable with those after fresh ET, with potentially better obstetric and perinatal outcomes. Therefore, it is time to avoid fresh ET in IVF, freeze-all available embryos and replace them in subsequent cycles? In this article, the authors have appraised the evidence underpinning this idea, exploring the biological plausibility of the concept and considering the implications of adopting such a strategy in routine clinical practice.
Avoiding fresh ET and freezing all embryos destined for transfer could improve the safety and effectiveness of IVF and intracytoplasmic sperm injection. The prospect of improved feto-maternal outcomes is particularly relevant given the increasing uptake of IVF across the world. The available evidence does not justify a change in practice at present but strongly supports the need for a large multicentre, randomised trial to evaluate the clinical and cost-effectiveness, as well as the acceptability of elective cryopreservation versus fresh ET. The nature of the proposed strategy poses major logistic challenges, both in terms of mounting a definitive trial, as well as implementing any policy changes that emerge from it.
Although freeze-all has several advantages over fresh cycles, one must remember that evidence is still lacking for using it in all patients. Moreover, there are many women who become pregnant and do not experience any obstetrical or perinatal complications even after a fresh ET. Elective cryopreservation of all embryos with transfer in subsequent FET cycles may be requited in cases who are at risk of developing OHSS and those who have high progesterone levels on the day of hCG trigger, but currently it is debatable whether a freeze-all strategy will benefit normal and poor responders.
Moreover, there is a need for further studies comparing the costs and cumulative PR between the two strategies. When advocating freeze-all, discussions regarding the pros and cons of the therapy should be held with the patient, including potential costs, delays in treatment, and potential risks associated with this strategy.
It is important to identify the subgroup of patients that would benefit from a freeze-all approach and it is the authors’ view that, based on current evidence and their practice, a freeze-all approach should be recommended only for those patients who are at risk for developing OHSS, undergoing PGS or PGD for genetic analysis, PCOS women, and those with elevated progesterone level on the day of hCG trigger. In an era of tailor-made therapies, a one-size-fits-all approach is inappropriate and the data to date do not support a shift to freeze-only cycles for all patients.
For an optimal ET technique, the use of soft catheters and performing the procedure under ultrasound guidance will improve results by making it less traumatic, standardised, and technically precise.