Meeting Summary
This symposium took place on the first day of the 2024 European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain. The goal was to present recommendations for treatment strategies and sequencing for patients with oestrogen-receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-), advanced/metastatic breast cancer after first-line (1L) therapy with endocrine therapy (ET) plus inhibitors of cyclin-dependent kinases 4 and 6 (CDK4/6i).
An expert panel of clinicians explained that most patients will eventually develop resistance to ET regimens during the advanced/metastatic setting, and they discussed the current ESMO recommendations for second- or later-line (2L+) treatment, which are driven by endocrine sensitivity status and biomarkers. Trial data that support the therapeutic recommendations in this patient population were presented, and the benefits and risks associated with different treatment options were summarised.
The panel emphasised the importance of testing for emergent ESR1 mutations at each progression during the advanced/metastatic treatment course, ideally by analysing circulating DNA from a liquid biopsy, in order to identify patients for whom elacestrant will be particularly beneficial.
The Treatment Landscape for ER+/HER2- Advanced/Metastatic Breast Cancer
Over 70% of breast cancers are ER+/HER2-, for which the backbone of treatment is ET.1-3 Advanced breast cancer (aBC) can be considered to include both inoperable, locally advanced breast cancer and metastatic breast cancer (mBC). While aBC/mBC remains largely incurable, important advances over the past 20 years have improved overall survival in patients with ER+/HER2- disease.
Virginia Kaklamani, Professor of Medicine in the Division of Hematology/Oncology at the University of Texas Health Sciences Center, San Antonio, USA, and leader of the breast cancer programme at the Mays Cancer Center, San Antonio, USA, explained that treatment choices for patients with ER+/HER2- mBC are affected by the complexity and heterogeneity of the disease, the characteristics of the individual patient (e.g., performance status, imminent organ failure, menopausal status, and prior lines of therapy), and the genomic landscape in terms of endocrine sensitivity/resistance and biomarkers (Figure 1).2-5
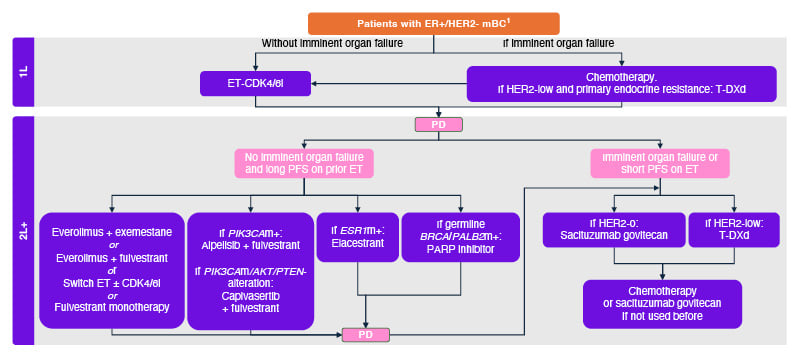
Figure 1: Treatment choices are driven by endocrine sensitivity status and biomarkers.
Adapted from Gennari A et al.2 2021, and ESMO Metastatic Breast Cancer Living Guidelines 2023.3
1L: first line; 2L+: second and later lines; mBC: metastatic breast cancer; BRCA: breast cancer gene; CDK4/6i: cyclin-dependent kinase 4/6 inhibitor; ER: oestrogen receptor; ESR1: oestrogen receptor 1; ET: endocrine therapy; HER2: human epidermal growth factor receptor 2; m: mutation; PALB2: partner and localiser of BRCA2; PARP: poly(ADP-ribose) polymerase; PD: progressive disease; PFS: progression-free survival; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; T-DXd: trastuzumab deruxtecan.
Treatment Choices at First-Line
The 1L standard of care (SoC) in ER+/HER2- mBC is ET plus CDK4/6i.2,6,7 ETs used for this indication include aromatase inhibitors (AI), such as anastrozole, letrozole, and exemestane; and selective oestrogen receptor degraders (SERD), such as fulvestrant.8 The addition of a CDK4/6i such as palbociclib, ribociclib, or abemaciclib to ET provides significant benefits both in terms of progression-free survival (PFS) and overall survival (OS) through the suppression of cell proliferation.9-14 The median duration of treatment with SoC at 1L is approximately 15–22 months (based on pivotal trials).12,15,16
Median PFS (mPFS) in the PALOMA-2 (palbociclib plus letrozole), MONALEESA-2 (ribociclib plus letrozole), MONALEESA-7 (ribociclib plus ET), and MONARCH-3 (abemaciclib plus non-steroidal AI) trials was 24.8 months (95% CI: 22.1 to not estimable), 25.3 months (95% CI: 23.0–30.3), 23.8 months (95% CI: 19.2 not reached [NR]), and 28.2 months (95% CI: not reported), respectively,9-12 with a median overall survival (mOS) of 53.8 months (95% CI: 49.8–59.2), 63.9 months (95% CI: 52.4–71.0), 58.7 months (95% CI: not reported), and 63.7 months (95% CI: not reported), respectively.13-15,17
As these data imply, most patients will eventually develop resistance to ET.2,4 Kaklamani explained that in ER+/HER2- aBC/mBC, resistance to ET can be classified by clinical and molecular variables. In clinical terms, ET resistance in the aBC/mBC setting can be considered primary (disease progression within the first 6 months of 1L ET-based therapy) or secondary (disease progression after more than 6 months of 1L ET-based therapy, or after any duration of 2L+ ET therapy).4,18 In molecular terms, ET resistance can be considered to be intrinsic (e.g., alterations of the PI3K/AKT/mTOR, RAS-MAPK pathway or fibroblast growth factor receptor 1 pathway, or mutations in BRCA1/2, RB1, or TP53) or acquired mechanisms of resistance (e.g., ESR1 mutations, occurring after prior ET in aBC/mBC).4,19-21
Kaklamani emphasised that different treatment mechanisms are effective for different mechanisms of resistance (Kaklamani, personal communication).
Treatment Choices at Second- or Later-Line are Driven by Endocrine Sensitivity, Biomarker Status, and Toxicity
In patients with ER+/HER2- mBC who do not have imminent organ failure and who experience disease progression after a long PFS on prior ET plus CDK4/6i (suggesting continued ET sensitivity), guidelines recommend exhausting ET options.2,3,6 Sequential ET in combination with a CDK4/6i, mTOR inhibitor (everolimus), PIK3 inhibitor (alpelisib), AKT inhibitor (capivasertib), or ET monotherapy are therefore used at 2L+ in this population.2,3,6 However, Kaklamani stressed that there remains a considerable margin for therapeutic improvement.
For example, ET monotherapies only provide an mPFS of around 2–4 months.22-25 In addition, combination therapies such as ET plus CDK4/6i or ET plus PI3K/AKT/mTOR inhibitors can be associated with toxicity. For example, CDK4/6i are associated with adverse events (AE) such as neutropenia, leukopenia, and anaemia, and sometimes with diarrhoea,16,26,27 with discontinuation due to AEs in up to 19% of patients,16,26,28 and PI3K/AKT/mTOR inhibitors are associated with AEs such as diarrhoea, rash, hyperglycaemia, and stomatitis,29-31 with discontinuation rates due to AEs in up to 24% of patients.32-34 As an intramuscular injection, monotherapy or combination therapy with fulvestrant can also be associated with injection site pain, as well as musculoskeletal pain, back pain, and peripheral neuropathy.35
Mutations in key genes are used as therapeutically relevant biomarkers to guide treatment choices. For example, mutations in the genes PIK3CA, ESR1, or BRCA/PALB2.2,3
Treatment choices at second- or later-line for patients without specific biomarkers
In the absence of specific mutation biomarkers, ESMO guidelines for 2L+ treatment of ER+/HER2- mBC (without imminent organ failure and with a long PFS on prior ET) include switching ET and/or CDK4/6i, combining everolimus with either fulvestrant or exemestane, or fulvestrant monotherapy.2,3
Unfortunately, rechallenge with a CDK4/6i has been associated with mixed results in clinical trials. Positive findings were reported in the MAINTAIN trial for ribociclib plus fulvestrant/exemestane versus fulvestrant/exemestane monotherapy (mPFS: 5.3 months versus 2.8 months, respectively).36 A statistically significant, but not clinically meaningful, efficacy improvement was also found with abemaciclib plus fulvestrant versus fulvestrant monotherapy in the postMONARCH study (mPFS: 6.0 months versus 5.3 months, respectively), though benefits were not observed in patients with prior ribociclib therapy in the latter study.37,38 However, no significant improvements were reported from palbociclib plus fulvestrant versus fulvestrant monotherapy in the PACE trial (mPFS: 4.6 months versus 4.8 months, respectively),39 or from palbociclib plus fulvestrant/letrozole versus fulvestrant/letrozole monotherapy in the PALMIRA study (mPFS: 4.2 months versus 3.6 months, respectively).40
Though the combination of an mTOR inhibitor plus fulvestrant/exemestane has shown positive results in the overall ER+/HER2- aBC/mBC population at 2L+, patients with an ESR1 mutation appear to receive less benefit.41-45 For example, the BOLERO-2 trial of everolimus plus exemestane versus exemestane monotherapy, conducted in a population not previously exposed to CDK4/6 inhibitors, was associated with an mPFS of 7.8 months versus 3.2 months across all patients, yet an mPFS of 5.4 months versus 2.8 months among patients with an ESR1 mutation.44,45
Treatment choices at second- or later-line for patients with AKT/PIK3CA/PTEN alterations
ESMO guidelines recommend 2L+ treatment with fulvestrant plus alpelisib for patients with ER+/HER2- mBC (without imminent organ failure and with a long PFS on prior ET) who are positive for a pathogenic mutation in PIK3CA (PIK3CAmut) and who have prior exposure to an AI.2,3
Fulvestrant plus alpelisib is approved for use in patients with ER+/HER2- PIK3CAmut aBC/mBC after disease progression following endocrine monotherapy.29 This is based on results from the SOLAR-1 study, which enrolled patients with prior AI therapy (only 6% had received prior CDK4/6i therapy) into two cohorts based on tumour-tissue PIK3CA mutation status.46 In the PIK3CAmut cohort, 169 patients received alpelisib plus fulvestrant, and 172 patients received placebo plus fulvestrant. Over the course of the trial, the mPFS was 11 months in the alpelisib-fulvestrant group versus 5.7 months in the placebo-fulvestrant group, with a hazard ratio (HR) of 0.65 (95% CI: 0.50–0.85; p<0.001).46
However, Kaklamani pointed out that analysis of the subsequent BYLieve study, in which all patients had prior CDK4/6i therapy, alpelisib-fulvestrant tended to be less effective (overall mPFS: 8.0 months).47 The mPFS with alpelisib-fulvestrant was just 5.6 months in the ESR1mut group (n=27), compared with 8.3 months in the wild-type ESR1 group (n=75).47
The AKT kinase inhibitor, capivasertib, has also been approved for use in combination with fulvestrant, in ER+/HER2- aBC/mBC with one or more PIK3CA, AKT1, or PTEN mutations following recurrence/progression on ET.31 In the CAPItello-291 study, patients with aBC/mBC and mutations in PIK3CA, AKT1, or PTEN had an mPFS of 7.3 months with capivasertib-fulvestrant (n=155) versus 3.1 months with placebo-fulvestrant (n=134), with an adjusted HR of 0.5 (95% CI: 0.38–0.65; p<0.001).25
Kaklamani noted that subgroup analyses showed that mPFS was shorter for both capivasertib-fulvestrant and placebo-fulvestrant in patients with prior CDK4/6i exposure (5.5 months versus 2.6 months), and shorter still with prior chemotherapy for aBC/mBC (3.8 months versus 2.1 months), or with liver metastases at baseline (3.8 months versus 1.9 months).48 Data on the efficacy of capivasertib-fulvestrant in patients with ESR1mut is not available.
Treatment choices at second- or later-line for patients with BRCA/PALB2 mutation
ESMO guidelines recommend the consideration of 2L+ treatment with poly ADP ribose polymerase inhibitor (PARPi) monotherapy (olaparib or talazoparib) for patients with ER+/HER2- mBC (without imminent organ failure and with a long PFS on prior ET) with a pathogenic germline mutation in BRCA1/2 (BRCAmut) or PALB2 (PALB2mut).2,3
In the OlympiAD study, patients with HER2- mBC and germline BRCA1/2mut, and up to two prior chemotherapy regimens for metastatic disease, received olaparib (n=205) or the physician’s choice of chemotherapy (n=97).49 The mPFS was 7.0 months in the olaparib group versus 4.2 months in the chemotherapy group, with an adjusted HR of 0.58 (95% CI: 0.43–0.80; p<0.001).49
A similar benefit of PARPi over chemotherapy was reported in the EMBRACA study, with an mPFS of 8.6 months in patients treated with talazoparib (n=287) and 5.6 months in patients treated with chemotherapy (n=144), with an adjusted HR of 0.54 (95% CI: 0.41–0.71; p<0.001).50
PALB2, like BRCA1/2, is involved in DNA repair, and some limited data have confirmed that PARPi are likely to have a benefit in patients with PALB2mut.51,52 However, because of the low frequency of the PALB2 mutation, dedicated studies may not be possible.53
Summary of treatment options at second- or later-line
Kaklamani emphasised that the use of fulvestrant monotherapy or ET combination therapy appears to be associated with a consistently lower PFS duration in patients with prior CDK4/6i therapy than in those without, and PFS duration appears to be lower still in those patients who also harbour an ESR1 mutation.15,22,25,29,30,36-38,42,47,48,54-61
Treatment choices at second- or later-line for patients with ESR1 mutation
ESMO guidelines recommend elacestrant at 2L for patients with ER+/HER2- mBC (without imminent organ failure and with a long PFS on prior ET) who harbour a mutation in ESR1 (ESR1mut) and experience disease progression after at least 1 line of ET.2,3
This recommendation is based on results from the EMERALD study, which enrolled 477 patients with ER+/HER2- aBC/mBC who had progressed/relapsed after 1–2 lines of ET for aBC/mBC, one of which had to be combined with a CDK4i.22 Patients were stratified by ESR1mut status and were randomised 1:1 to treatment with elacestrant (n=239) or the investigator’s choice of SOC (an AI or fulvestrant; n=238) until disease progression. Kaklamani stressed that all patients in the trial had received prior CDK4/6i therapy, and that across the elacestrant group and the SOC group, 68% and 71% of patients had visceral metastases, respectively, and 48% and 47% were ESR1mut, respectively.22
Among patients with ESR1mut, elacestrant (n=115) versus SOC (n=113) was associated with a 45% reduction in the risk of progression or death (HR: 0.55; 95% CI: 0.39–0.77; p=0.0005). The 6-month PFS in the elacestrant group versus the SOC group was 40.8% versus 19.1%, respectively, and the 12-month PFS was 26.8% versus 8.2%, respectively.22
As EMERALD patients population included primary endocrine resistance, an exploratory analysis showed that duration of prior ET plus CDK4/6i therapy may be positively associated with mPFS in patients with ESR1mut. Among patients with ≥6 months of prior ET plus CDK4/6i, mPFS with elacestrant (n=103) was 4.1 months, compared with 1.9 with SOC (n=102). However, among patients with ≥12 months of prior ET plus CDK4/6i, mPFS reached 8.6 months (n=78) versus 1.9 months (n=81), respectively; and in those with ≥18 months of prior ET plus CDK4/6i, mPFS reached 8.6 months (n=55) versus 2.1 months (n=56), respectively.38 The clinically meaningful improvement in PFS versus SOC in patients with longer prior exposure to prior ET plus CDK4/6i has been demonstrated regardless of the metastatic site location or number; coexistence of PIK3CAmut, TP53mut, or HER2-low expression; or ESR1mut variant (Table 1).38 Kaklamani explained that, because the benefit observed with elacestrant versus SOC was not impacted by other commonly coexisting mutations or molecular expressions, it is highly likely that ESR1 mutations were the main driver of disease in this population.
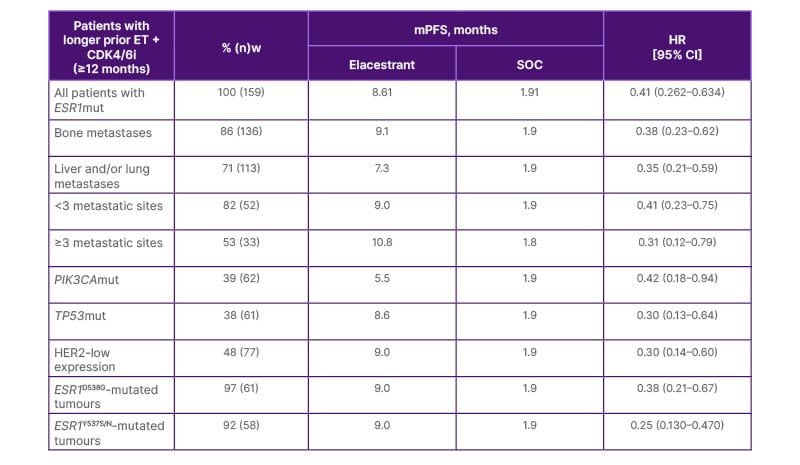
Table 1: PFS in subgroups of patients with ESR1-mutated tumours and longer prior ET+CDK4/6i.
Adapted from Bardia A et al.38 2024.
CDK4/6i: cyclin dependent kinase 4/6 inhibitor; CI: confidence interval; ESR1: oestrogen receptor 1; ET: endocrine therapy; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; mPFS: median progression-free survival; mut: mutation; PIK3CA: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; SOC: standard of care; TP53: tumour protein 53.
To explain the significance of these findings, Kaklamani stressed that if a patient has received ≥12 months of prior ET plus CDK4/6i before experiencing disease progression, their tumour is likely to be endocrine sensitive, whereas the tumour of a patient with disease progression after, for example, 4 months of prior ET plus CDK4/6i therapy is likely to be endocrine resistant. Ultimately, clinicians need to select tumours that are endocrine sensitive to have confidence in further ET, and longer PFS with prior exposure to ET+CDK4/6i (>6 months)18 is a good guideline to use (Kaklamani, personal communication).
In the overall EMERALD population, the majority of adverse events that occurred were Grade 1 or 2;22 no Grade 4 treatment-related AEs were reported.62 In the elacestrant and SOC arms of the study, 3.4% and 0.9% of patients discontinued treatment due to treatment-related AEs.22 Nausea was responsible for elacestrant discontinuation in 1.3% of patients, though Kaklamani pointed out that the use of antiemetics in the elacestrant group was actually less than in the SOC (AI) group.38 No haematologic safety signal was observed, and none of the patients in either treatment arm had sinus bradycardia.38
Kaklamani stressed that elacestrant is not the only endocrine-based therapy being developed for patients with ER+/HER2- mBC, and that data for other drugs are expected in the next future (Kaklamani, personal communication).
Treatment choices at second- or later-line for patients with imminent organ failure or primary endocrine resistance
For those patients with ER+/HER2- mBC who have imminent organ failure or who had a short PFS (<6 months) on ET at 1L (indicative of primary endocrine resistance), ESMO guidelines recommend 2L+ treatment with chemotherapy-based regimens at 2L+.2,3,18
In the recent DESTINY-Breast06 study, T-DXd was evaluated in patients with HER2-low or -ultralow after disease progression on ET (≥2 prior lines of ET or 1 line of ET and primary endocrine resistance) but with no prior chemotherapy for mBC.63 Among patients with HER2-low, the mPFS in the T-DXd group (n=359) was 13.2 months, while the mPFS in the physician’s choice of chemotherapy group (n=354) was 8.1 months, indicating that T-DXd significantly improved PFS versus treatment with physician’s choice of chemotherapy (HR: 0.62; 95% CI: 0.51–0.74; p<0.0001).63
Sacituzumab govitecan, an antibody-drug conjugate consisting of a trop-2-directed antibody and a topoisomerase inhibitor, should be considered for patients in this population with HER2-0 and ≥2 prior lines of chemotherapy.3,64 In patients with HER2-low and ≥1 prior line of chemotherapy, trastuzumab deruxtecan (T-DXd), an antibody drug conjugate consisting of an HER2-directed antibody and a topoisomerase inhibitor, should be considered.2,3,65
These data demonstrate that ADCs should be considered for patients with ER+/HER2- mBC who have imminent organ failure or who had a short PFS (<6 months ) in a prior line of endocrine therapy, or are no longer eligible for endocrine therapy-based regimens.
Biomarkers of Acquired Resistance in Breast Cancer
Frederik Marmé, Professor of Experimental and Translational Gynecologic Oncology at University Hospital Mannheim, Germany, and co-chair of the AGO Study Group, explained that breast cancer is a dynamic disease in which mutations may emerge over the course of 1L mBC treatment.2-5
One of the key mechanisms of resistance to ET is the emergence of mutations in the ESR1 gene.21 Marmé stressed that because ESR1 mutations are acquired during 1L mBC treatment, they are sub-clonal, which means that the molecular profile can vary between and within tumour sites.4,5
Mutations in ESR1 that alter the ligand-binding domain of the oestrogen receptor result in constitutive activation of the oestrogen receptor, which confers ligand independence.20,66 Constitutive oestrogen receptor signalling leads to increased proliferation, differentiation, and survival in the affected cancer cells.67,68 ESR1 mutations have been associated with endocrine resistance, visceral metastases, and poorer outcomes. 21,67-70
ETs exert their anti-tumour activity by binding to the ligand-binding pocket of the oestrogen receptor and inhibiting the activation of downstream targets.21 By altering the ligand-binding domain, ESR1 mutations can induce resistance to ETs.21
Marmé explained that the longer mBC is exposed to ET, the greater the risk of developing ESR1 mutations during treatment, which eventually emerge in up to 40% of patients (Figure 2).21,22,71-77
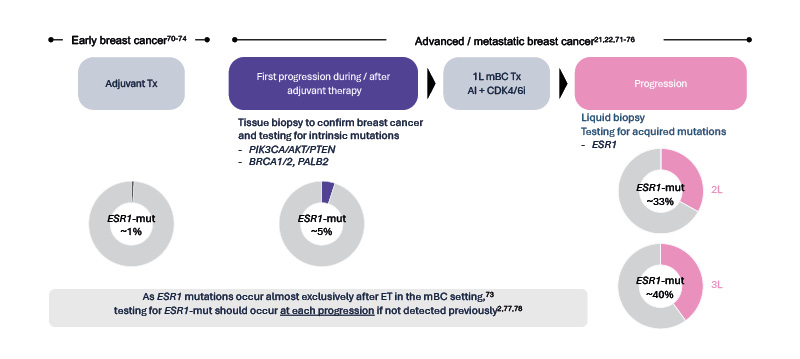
Figure 2: Longer exposure to ET in mBC increases the chance of developing ESR1mut during treatment.2,21,22,70–78
Figure courtesy of Menarini Stemline.
1L: first-line; 2L: second-line; 3L: third-line; ESR1: oestrogen receptor 1; ET: endocrine therapy; mBC: metastatic breast cancer; mut: mutation; Tx: treatment.
As described above, ESR1 mutations drive treatment decisions because the biomarker profile of ER+/HER2- mBC influences the choice of therapy in 2L+.2 In light of this, ESMO, NCCN, and ASCO recommend testing for ESR1 mutations at each progression if not detected previously.79-83
Unlike BRCA and PIK3CA mutations, ESR1 mutations are typically undetectable in the primary tumour because they are sub-clonal, and archival tissue from the primary tumour should not be used to identify ESR1mut.79,84 For this reason, testing of ESR1mut is best performed in liquid biopsy (ctDNA).79,83 Blood-based ctDNA is also preferred for ESR1mut testing because it is more sensitive for these mutations compared with tissue sampling. For example, the ESR1mut prevalence rate in liquid biopsy is higher than in tissue, especially when ctDNA tumour fraction is ≥1.85 Kaklamani described the interpretation of ctDNA results as relatively straightforward, especially when the laboratory performing the test provides support.
What About Patients with a Tumour That is Positive for More Than One Biomarker?
Marmé explained that, in a tumour with ESR1mut that is also positive for other biomarkers, thinking about ctDNA allele frequency is not particularly useful when making treatment decisions. He emphasised that intrinsic mutations such as PIK3CA or AKT would likely have a higher allelic frequency than an acquired ESR1 mutation, which will probably have occurred in a sub-clone of the original tumour. However, he stressed that elacestrant will also work in those tumour cells that express wildtype ESR1, and it is important to use a treatment that will effectively treat the ESR1mut cells (Marmé, personal communication).
Kaklamani added that, if there are more than one treatment option available for a patient with co-mutations, she would consider the toxicity of the available treatments, and in general, single-agent ET tends to be better tolerated than combination therapies (Kaklamani, personal communication).
Closing Remarks
Peter Schmid, Chair of Cancer Medicine at Barts Cancer Institute, Queen Mary University, and Clinical Director of the Breast Cancer Centre at the St. Bartholomew Cancer Centre, Barts Hospital, London, UK, concluded the symposium by emphasising the key takeaways.
Essentially, a biomarker-driven treatment algorithm is needed to ensure optimal treatment selection for patients with ER+/HER2- mBC.6,12,22,25,47 Biomarkers include intrinsic mutations in BRCA or PIK3CA and acquired ESR1 mutations that emerge over time in up to 40% of patients after ET.21,22,76,77,86 Longer PFS on prior ET + CDK4/6i can be used as a surrogate for endocrine sensitivity in ESR1mut tumours,22,38,87 and endocrine-sensitive tumours with coexisting PIK3CA and ESR1 mutations may benefit from elacestrant monotherapy before PI3K/AKT inhibitors, as data suggest the endocrine receptor pathway may drive disease progression in these patients.38
Schmid explained that fundamentally, at 1L progression, patients with ER+/HER2- aBC/mBC should be tested for genomic alterations in liquid biopsy to define the optimal treatment.2,3







