Presenter: Andrew X. Zhu1,2
1. Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, Massachusetts, USA
2. Jiahui International Cancer Center, Jiahui Health, Shanghai, China
Disclosure: Dr Zhu has participated in advisory boards for Bayer, Eisai, Exelixis, Lilly, Merck, Roche-Genentech, and Sanofi.
Acknowledgements: Medical writing assistance was provided by Dr Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Support: This article was funded by an educational grant from Agios Pharmaceuticals, Inc. The views and opinions expressed are those of the speaker and not necessarily of Agios Pharmaceuticals, Inc.
Citation: EMJ Oncol. 2021;9[Suppl 2]:2-5.
Meeting Summary
Cholangiocarcinoma (CCA) is a rare cancer for which there are limited effective therapies.1,2 Mutations in the isocitrate dehydrogenase 1 gene (IDH1) occur in up to 20% of intrahepatic CCA3 and lead to the production of the oncometabolite D-2-hydroxyglutarate, which promotes oncogenesis. Ivosidenib is a first-in-class, oral, small molecule inhibitor of mutant IDH1 (mIDH1).4 The global Phase III ClarIDHy5 study assessed the efficacy of ivosidenib versus placebo in patients with previously treated advanced IDH1-mutant CCA.
Final Results from ClarIDHy, a Global, Phase III, Randomised, Double-Blind Study of Ivosidenib Versus Placebo in Patients with Previously Treated Cholangiocarcinoma and an Isocitrate Dehydrogenase 1 (IDH1) Mutation
Doctor Andrew X. Zhu
In an oral presentation at the 2021 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology (ASCO GI 2021), Dr Zhu introduced ClarIDHy as the first global, randomised, double-blinded, placebo-controlled, Phase III study of a targeted, oral therapeutic with a noncytotoxic mechanism of action (ivosidenib) in advanced mIDH1 CCA.6,7 Adults with unresectable or metastatic mIDH1 CCA who had received one or two prior therapies were randomised 2:1 to ivosidenib 500 mg once daily orally in continuous 28-day (+2 days) cycles (n=126) or matched placebo (n=61).6,7 Crossover from placebo to ivosidenib was permitted at radiographic disease progression.6,7
Most patients had intrahepatic CCA at diagnosis (113/126 [89.7%] and 58/61 [95.1%] in the ivosidenib and placebo groups, respectively) and metastatic disease at screening (117/126 [92.9%] and 56/61 [91.8%], respectively). The most common IDH1 mutation was R132C (86/126 [68.3%] patients on ivosidenib and 45/61 [73.8%] on placebo).
As of 31st May 2020, a large proportion of placebo-treated patients (43/61 [70.5%]) had crossed over to open-label ivosidenib upon radiographic disease progression and unblinding, as permitted by the study protocol. The remaining 18 placebo patients (29.5%) did not cross over (never dosed [n=2], took the wrong drug [n=1], received another treatment [n=1]; or death as end of study reason [n=12], withdrawal of consent as end of study reason [n=2]).
Dr Zhu highlighted that the median treatment duration was 2.8 months in the ivosidenib arm versus 1.6 months in the placebo arm. After crossover from placebo, median treatment duration on ivosidenib was 2.7 months. Notably, the upper limit of the range of treatment duration was nearly 5-fold higher for ivosidenib compared with placebo (34.4 months in the ivosidenib arm versus 6.9 months in the placebo arm; 29.8 months on ivosidenib in placebo patients after crossover). A total of 25 patients (15.1%), including six who crossed over from placebo, remained on ivosidenib for ≥1 year.
Progression-free survival (PFS; by blinded independent radiology centre), the primary endpoint, was significantly improved with ivosidenib (median: 2.7 months; 95% confidence interval [CI]: 1.6–4.2) compared with placebo (median: 1.4 months; 95% CI: 1.4–1.6), with a hazard ratio [HR] of 0.37 (95% CI: 0.25–0.54; one-sided p<0.0001).7 PFS results were consistent across all subgroups analysed, including sex, type of cancer, and extent of disease at screening, and favoured ivosidenib.7
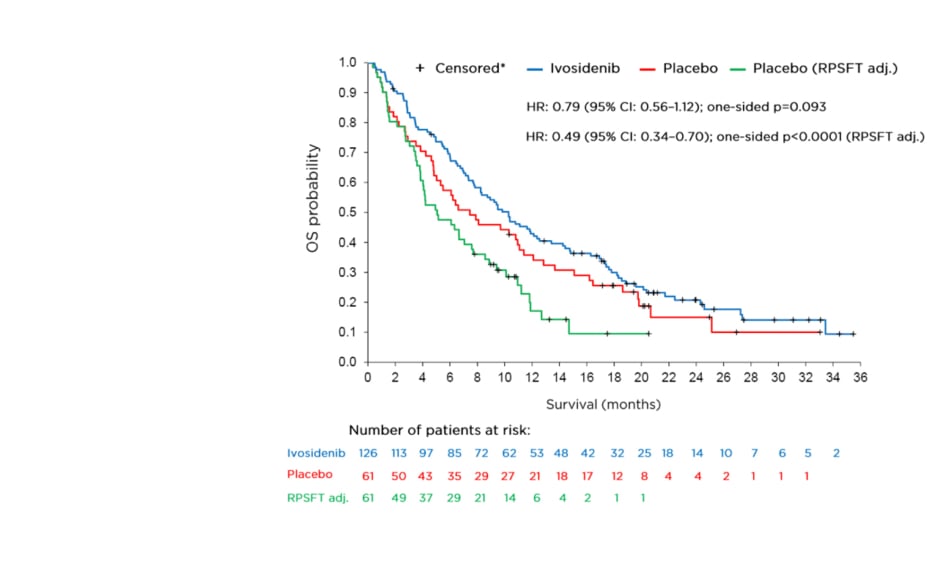
The final analysis for overall survival (OS), a key secondary endpoint, is shown in Figure 1. The OS analysis numerically favoured ivosidenib despite a high rate of crossover from the placebo arm (approximately 70%) but was not statistically significant (HR: 0.79; 95% CI: 0.56–1.12; one-sided p=0.093). To adjust for the effect of crossover from placebo to ivosidenib, the rank-preserving structural failure time (RPSFT)8-10 model was implemented as a prespecified analysis. Dr Zhu stated that a highly statistically significant improvement in median OS was observed with ivosidenib compared with placebo in this RPSFT- adjusted analysis (HR: 0.49; 95% CI: 0.34–0.70; one-sided p<0.0001). The median OS for ivosidenib was 10.3 months compared with 7.5 and 5.1 months, respectively, for placebo before and after adjustment for crossover.
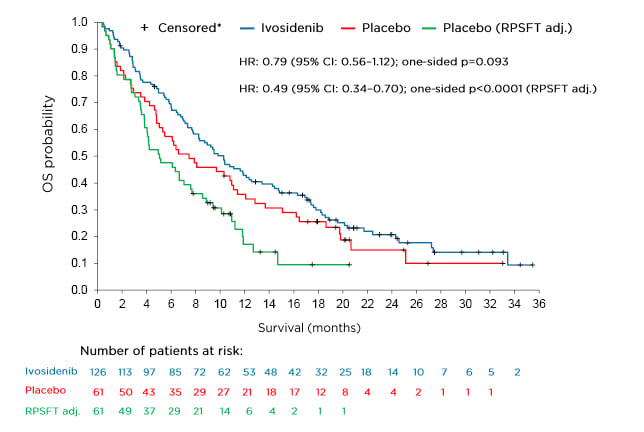
Figure 1: Final overall survival analysis.
*Patients without documentation of death at the data cut-off date were censored at the date the patient was last known to be alive or the data cut-off date, whichever was earlier.
CI: confidence interval; HR: hazard ratio; OS: overall survival; RPSFT adj.: rank-preserving structural failure time adjusted analysis.
Table 1 shows treatment-emergent adverse events (TEAE) that occurred at a rate >15%. According to Dr Zhu, TEAE were consistent with those in the preceding Phase I study.11 The percentage of patients who experienced any TEAE was similar across treatments (97.6% [120/123] for ivosidenib, 96.6% [57/59] for placebo), and for the group that included all patients initially randomised to ivosidenib plus the 43 patients who crossed over to ivosidenib (97.0% [161/166] for total ivosidenib). The most common TEAE was nausea (51/123 [41.5%] for ivosidenib, 63/166 [38.0%] for total ivosidenib, 17/59 [28.8%] for placebo).
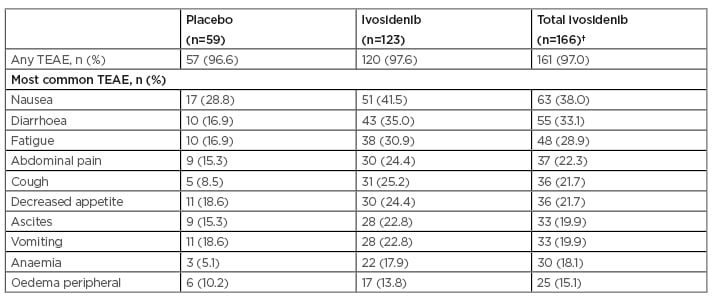
Table 1: Treatment-emergent adverse events occurring at a rate of >15%.*
*>15% cut-off used for all grade TEAE based on total ivosidenib.
†Total ivosidenib includes 43 patients initially assigned to placebo who had crossed over to ivosidenib upon radiographic disease progression and unblinding. All randomised patients as of 31st May 2020.
TEAE: treatment-emergent adverse event.
The most common Grade ≥3 TEAE for total ivosidenib versus placebo were ascites (9.0% versus 6.8%), anaemia (7.2% versus 0.0%), and increased blood bilirubin (5.4% versus 1.7%).
Dr Zhu observed that ivosidenib preserved certain patient-reported, health-related quality of life (HRQoL) subscales, including physical functioning, which was prespecified in the statistical analysis plan.12 Ivosidenib preserved European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) Physical Functioning scale, whereas placebo patients experienced decline from baseline at Cycle 2 Day 1 (two-sided p=0.002) and Cycle 3 Day 1 (two-sided p=0.004). Ivosidenib was also favoured on the prespecified QLQ-C30 Pain subscale at Cycle 2 Day 1 (two-sided p=0.039). Neither arm was favoured on other prespecified subscales (QLQ-C30 Appetite Loss; or the cholangiocarcinoma and gallbladder cancer module [QLQ-BIL21] Pain and Eating; all two-sided p>0.05). For the non-prespecified subscales of the QLQ-C30 and QLQ-BIL21 instruments in which significant differences were observed, all results favoured ivosidenib.
In conclusion, Dr Zhu highlighted that ivosidenib was associated with a numerical improvement in OS compared with placebo despite a high rate of crossover from the placebo arm (approximately 70%). This finding was further supported by a statistically significant improvement in OS compared with placebo in the prespecified RPSFT model analysis to adjust for crossover. As previously presented,6,7 there was a statistically significant improvement in the PFS primary endpoint. Ivosidenib also had a tolerable safety profile and preserved aspects of HRQoL.
Overall, the final results from the ClarIDHy global Phase III study demonstrated improvements in OS and PFS coupled with a favourable safety profile and supportive HRQoL data. These results reinforce the clinical benefit of ivosidenib in this aggressive disease with high unmet need for new therapies.