Interviewees: Maximilian J. Hochmair,1 Martin Reck,2 Ignacio Gil-Bazo,3 Marina Garassino4
1. Department of Respiratory and Critical Care Medicine, Karl Landsteiner Institute of Lung Research and Pulmonary Oncology, Vienna, Austria
2. Thoracic Oncology and Clinical Trial Departments, LungenClinic Grosshansdorf, Grosshansdorf, Germany
3. Department of Oncology, Clínica Universidad de Navarra, Program of Solid Tumors, Center for Applied Medical Research (CIMA), Navarra Health Research Institute (IDISNA), Madrid, Spain
4. Medical Oncology Division, National Cancer Institute of Milan, Milan, Italy
Disclosure: Dr Hochmair has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Pfizer, Takeda, and Roche; and has had consulting or advisory roles with Boehringer Ingelheim, MSD, Novartis, Takeda, and Roche. Prof Reck has received honoraria and consultant fees from Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer-Ingelheim, Eli Lilly, Merck, MSD, Novartis, Pfizer, Roche, and Samsung. Dr Gil-Bazo has received speaker bureau or consultant fees from AstraZeneca, Bristol Myers Squibb, Guardant Health, and MSD; has received research support from Gobierno de Navarra, Ministerio de Economía y Competitividad (Gobierno de España), and Fundación Merck Salud; and has had consulting or advisory roles for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, MSD, Pfizer, Takeda, and Roche. Prof Garassino has received speaker bureau or consultant fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Incyte, Inivata, MSD, Novartis, Otsuka Pharma, Pfizer, Roche, Sanofi Aventis, Seattle Genetics, and Takeda; and has participated in clinical trials for or received research funding from AstraZeneca, Bayer, Blueprint Medicine, Bristol Myers Squibb, Celgene, Clovis, Eli Lilly, Exelixis, GlaxoSmithKline, Incyte, Merck KGaA, Merck Serono, MSD, Novartis, Otsuka Pharma, Pfizer, Roche, Spectrum Pharmaceuticals, Takeda, Tiziana Sciences, and United Therapeutics.
Support: The publication of this interview feature was supported by Eli Lilly.
Acknowledgements: Medical writing assistance was provided by Bronwyn Boyes, London, UK.
Citation: EMJ Oncol. 2021;9[Suppl 1]:2-12.
![]()
Corrigendum: Expanding Possibilities in EGFR-Mutated Advanced NSCLC: Interviews with Four Key Opinion Leaders
Authors: Maximilian J. Hochmair, Martin Reck, Ignacio Gil-Bazo, Marina Garassino
Original citation: EMJ Oncol. 2021;9[Suppl 1]:2-12.
Date correction published: 29.1.2021
In the article by Hochmair et al. in Suppl 1 of EMJ Oncology 9.1 (pages 2–12) for Figure 3 the treatment sequencing options for standard of care (SOC) are incorrect for osimertinib. For both ‘Past SOC’ and ‘Alternative SOC’, the sentence beneath ‘Osimertinib’ should read “If T790M- (50%) 5.0 months”. Figure 3 originally read “If T790M+ (50%) 10.0 months” incorrectly. This has now been updated.
The authors apologise for the error and any inconvenience caused.
![]()
WHAT ARE THE UNMET NEEDS IN TREATING NSCLC?
Doctor Maximilian Hochmair
Background and Epidemiology
Worldwide, there were approximately 2.10 million patients diagnosed with, and 1.77 million deaths caused by, lung cancer in 2018.10 Approximately 85% of these cases were NSCLC,11 and the majority of patients presented with advanced disease at diagnosis.12 Traditionally, NSCLC was classified by histology but the classification paradigm has evolved to incorporate molecular subtypes to guide treatment decision making. One molecular subtype, EGFR mutations, was discovered in 2004 to be present in a subset of patients with NSCLC,13,14 and prevalence has ranged from 50–60% in Asian populations,5,6,15 10–15% in European populations,3,4,16 and 22% in North American populations.17,18 The aetiology, clinicopathological features, prognosis, and treatment paradigms in patients with EGFR-mutated NSCLC are distinct when compared with other NSCLC molecular subtypes.7,13-15,17
Current Treatment
Historically, chemotherapy was recommended as the first-line treatment in patients with advanced NSCLC.19-21 However, chemotherapy has significant toxicity, the therapeutic effect is limited, and the clinical outcomes are modest (median OS time of 8–10 months and a 5-year survival rate of approximately 15%).22,23 Discovery of mutations within the kinase domain of the EGFR gene has enabled a new era of targeted therapy in lung cancer.14,15,24 EGFR-tyrosine kinase inhibitors (TKI) have shown significant efficacy, are associated with fewer side effects, and have improved quality of life for patients with EGFR-mutated NSCLC, particularly those harbouring the exon 19 deletion or L858R.8,9,25-33 Currently, there are five different EGFR-TKI available for first-line use in EGFR-mutated NSCLC. The first-generation EGFR-TKI bind competitively and reversibly to the ATP-binding site of the EGFR tyrosine kinase domain and include erlotinib and gefitinib. Second-generation EGFR-TKI include afatinib and dacomitinib, which can covalently and irreversibly inhibit the enzymatic activation of EGFR (T790M), wild-type EGFR, and activating EGFR mutations. The third-generation EGFR-TKI osimertinib selectively and irreversibly blocks EGFR (T790M) and activating EGFR mutations, but not wild-type EGFR.34-36 Since 2004, there has been a number of randomised studies clearly showing the benefits of targeted therapy.6,8,9,25-33
Unmet Needs Associated with Current Treatment
Dr Hochmair believes that the availability of three generations of EGFR-TKI with different clinical profiles has revolutionised the treatment of EGFR-mutated NSCLC. However, despite initial clinical response to EGFR-TKI, virtually all advanced EGFR-mutated NSCLC inevitably acquire resistance mechanisms and progress at some point during treatment with gefitinib, erlotinib, dacomitinib, afatinib, and osimertinib. The most common acquired resistance to first- and second-generation EGFR-TKI is via a unique gatekeeper mutation: the exon 20 point-mutation T790M in the ATP-binding site of EGFR.37 The T790M mutation activates wild-type EGFR and increases ATP affinity.37 The incidence of T790M ranges from 49–63%.38-41 Other mechanisms of secondary resistance include activation of HER2, MET, PIK3CA, BRAF, NF1, FGFR, AXL, and the hedgehog signalling pathway.41 There are currently no further targeted therapy options available following osimertinib failure, and most patients will proceed to chemotherapy.22,42 Thus, there is an unmet need in EGFR-mutated advanced NSCLC for new treatment strategies, such as drug combinations, to delay the emergence of acquired resistance and disease progression.9
Rationale of Dual Blockade Treatment for EGFR-Mutated Advanced NSCLC
Angiogenesis and neovascularisation are critical for the growth, progression, and metastasis of NSCLC. Therefore, angiogenic pathways, including vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet-derived growth factor, are important biologic targets for tumour growth inhibition. Targeted therapies include neutralising antibodies to VEGFA (bevacizumab) and VEGF receptor 2 (VEGFR2) (ramucirumab), as well as receptor TKI that have preferential selectivity for the VEGFR.43 Preclinical studies have demonstrated that dual blockade of the EGFR and VEGF pathways improved antitumour activity compared with inhibition of the EGFR pathway alone. Several clinical trials have shown promising results with the combination of the anti-VEGFA antibody bevacizumab with an EGFR-TKI; however, conclusions in these trials were limited by small sample sizes, Asian-only populations, and open-label designs.9,42 More recently, the RELAY study demonstrated the benefit of dual blockade treatment with the monoclonal antibody ramucirumab in combination with the EGFR-TKI erlotinib. Ramucirumab is a human IgG1 antibody selective for VEGFR2 that inhibits binding of the VEGFA, VEGFC, and VEGFD ligands to VEGFR2. The RELAY study showed a significantly improved PFS with the combination of ramucirumab plus erlotinib versus erlotinib plus placebo as a front-line treatment for patients with EGFR-mutated NSCLC. The median PFS for the ramucirumab combination was higher than observed historically.6,9,25-33 These outcomes led Dr Hochmair to advocate the use of dual blockade with an antiangiogenic and an EGFR-TKI as first-line therapy in appropriate patients with EGFR-mutated NSCLC. He concluded: “The availability of three generations of EGFR-TKI with different clinical profiles has revolutionised the treatment of EGFR-mutated NSCLC. I believe that liquid-biopsy-based identification (versus cobas® [Roche Holding AG, Basel, Switzerland] EGFR-mutation testing) of EGFR T790M mutation-mediated resistance can better inform treatment sequencing decisions in EGFR-mutated NSCLC. I would advocate the use of ramucirumab plus erlotinib as first-line therapy in appropriate patients with EGFR-mutated NSCLC.”
WHAT DID THE RELAY CLINICAL TRIAL SHOW?
Professor Martin Reck
RELAY Trial Study Design
RELAY9 was a multicentre, double-blind, randomised clinical trial comparing an anti-VEGFR2 antibody plus a first-generation EGFR-TKI to the standard of care alone. The primary question Prof Reck wanted to answer was whether the combination regimen could improve efficacy in EGFR-mutated metastatic NSCLC. The study enrolled 449 patients in 100 centres in 13 countries. Adult patients with Stage IV NSCLC, confirmed EGFR exon 19 deletion or L858R mutation, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were eligible for enrolment. Patients were ineligible if they had a known EGFR T790M mutation, prior treatment with an EGFR-TKI or chemotherapy, or had brain metastases.
Patients were randomised in a 1:1 ratio to receive either combination therapy with ramucirumab 10 mg/kg every 2 weeks plus erlotinib 150 mg/day, or monotherapy with erlotinib 150 mg/day plus placebo. Patients were stratified by sex, geographical region, EGFR mutation type, and EGFR testing method.
The primary endpoint was investigator- assessed PFS in the intention-to-treat population. Independent PFS was also performed using a centralised radiological review of CT or MRI scans. Secondary endpoints included OS, overall response rate, duration of response (DoR), safety and toxicity, pharmacokinetics and immunogenicity, and patient-reported outcomes. Exploratory endpoints were PFS2 (defined as the time from randomisation to progression after a subsequent therapy) and the identification of biomarkers.
RELAY Trial Efficacy
At a median follow-up of 20.7 months, the investigator-assessed median PFS in the ramucirumab arm was 19.4 months (95% confidence interval [CI]: 15.4–21.6), which was significantly longer than the 12.4 months in the placebo arm (95% CI: 11.0–13.5; hazard ratio [HR]: 0.59; 95% CI: 0.461–0.760; p<0.0001) (Figure 1).9 The 1-year PFS also improved in the combination (ramucirumab plus erlotinib) group (71.9%; 95% CI: 65.1–77.6) compared with the placebo plus erlotinib group (50.7%; 95% CI: 43.7–57.3). The blinded independent radiological review (440 patients had evaluable scans) and analysis in the per-protocol population (437 evaluable patients) showed PFS results consistent with the primary investigator-assessed PFS analysis.
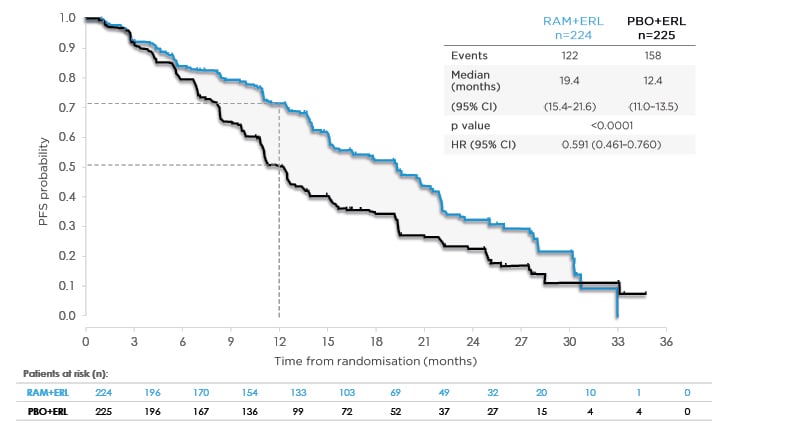
Figure 1: RELAY primary endpoint: investigator-assessed progression-free survival.
Data from Nakagawa K et al. Lancet Oncol. 2019;20(12):1655-1669.9
Consistent PFS benefit by independent, blinded central review (HR: 0.671; 95% CI: 0.518–0.869; p=0.0022).
CI: confidence interval; ERL: erlotinib; HR: hazard ratio; PBO: placebo; PFS: progression-free survival; RAM: ramucirumab.
In addition, DoR and PFS2 were improved with ramucirumab plus erlotinib compared with erlotinib plus placebo (median DoR: 18.0 months [95% CI: 13.9–19.8] versus 11.1 months [95% CI: 9.7–12.3], respectively, PFS2: HR: 0.690; 95% CI: 0.490–0.972).
This PFS benefit appeared not to be driven by an improvement in the overall response rate, which was not significantly different in the combination and monotherapy arms (76% versus 75%). OS data were immature at data cut-off for this analysis; however, at this stage, there was no significant difference between treatment groups. Prof Reck believes that, in the absence of mature OS data, the favourable PFS2 that incorporates the next line of therapy suggest the possibility of OS benefit.
At the time the data were presented, 29% (64/224) of patients in the combination arm and 19% (43/225) in the monotherapy arm were still receiving the study treatment.
RELAY Trial Study Safety
No new adverse events (AE) were identified in this study. The rate of treatment-emergent AE (TEAE) of at least Grade 3 was higher among the combination-treated than monotherapy-treated patients, at 72% and 54%, respectively (Table 1).9 Most of the TEAE could be addressed with treatment discontinuation or dose reduction. Hypertension was the most common TEAE; 24% experienced Grade 3 hypertension in the combination arm compared with 5% in the monotherapy arm, and there were no incidences of Grade 4 or 5 hypertension in either group. A comparable 13% in the combination group and 11% in the monotherapy group discontinued all study drugs because of TEAE (Table 1). The majority of the observed bleeding events that occurred were related to epistaxis and there was no increased rate of severe pulmonary bleeding events in the combination arm. Two deaths (0.9%) occurred in the combination treatment group: one was considered a related AE (haemothorax) and one was considered a TEAE not related to study treatment (encephalitis influenza). No deaths occurred in the monotherapy group.
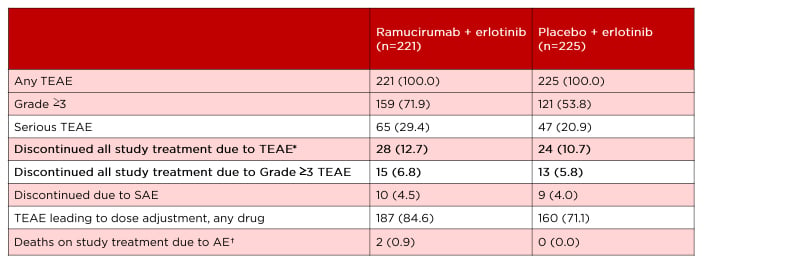
Table 1: RELAY safety overview.
Data from Nakagawa K et al. Lancet Oncol. 2019;20(12):1655-1669.⁹
*The most frequent reasons for discontinuation because of an AE were increased alanine aminotransferase (n=3, ramucirumab arm; n=4, placebo arm), paronychia (n=3, ramucirumab; n=0, placebo), and abnormal hepatic function (n=0, ramucirumab; n=3, placebo).
†Of the two deaths in the ramucirumab plus erlotinib group, one was considered a related AE (haemothorax) and one was considered a TEAE not related to study treatment (encephalitis influenza).
Data shown as: n (%).
AE: adverse event; SAE: serious adverse event; TEAE: treatment-emergent adverse event.
To Prof Reck, the most interesting result was that the PFS benefit in the ramucirumab arm was observed regardless of mutation type. This is the first study showing comparable efficacy rates in both mutation types, as commonly patients with L858R mutation have poorer outcomes.
In this study, most subgroups appeared to have a PFS advantage following the addition of ramucirumab to erlotinib. Furthermore, comparable and consistent efficacy was achieved in both East Asian (75% were Asian) and non-East Asian populations. The treatment-emergent, resistance-mediating EGFR T790M rates were also similar between groups (p=0.849); ramucirumab plus erlotinib (43%; 95% CI: 30–58%) and erlotinib plus placebo (47%; 95% CI: 36–58%).
Therefore, both groups could qualify for post-progression sequential osimertinib therapy. In summary, Prof Reck considers that the RELAY trial provided prospective confirmation that dual blockade with an antiangiogenic agent and an EGFR inhibitor is a viable and valuable first-line treatment strategy for patients with EGFR-mutated metastatic NSCLC. He summarised: “The RELAY regimen of ramucirumab plus erlotinib is, therefore, a viable new treatment option for the initial treatment of patients with EGFR-mutated advanced NSCLC.”
ARE ALL EGFR-MUTATIONS SIMILAR?
Doctor Ignacio Gil-Bazo
Common EGFR Mutations
NSCLC is associated with a poor prognosis:44 the 5-year survival for patients with EGFR-mutated NSCLC is 14.6%.45 The identification of specific actionable mutations and development of molecular profiling in advanced NSCLC has enabled more personalised therapeutic strategies and resulted in improved outcomes.18 EGFR, a member of the ErbB receptor TK family, plays an important role in the development and progression of NSCLC.46EGFR activates JAK, PI3K, reactive oxygen species, and Ras pathways and overexpression can cause cell proliferation, angiogenesis, tumour invasion, metastasis, and inhibition of cell apoptosis.47-49 The most common EGFR mutations are exon 19 deletion (45% of patients) and L858R mutations (40%).7 The ‘uncommon’ EGFR mutations account for 10–18% of all EGFR mutations and primarily consist of a heterogeneous group of genetic alterations (exon 18 G719, exon 21 L861, and exon 20 S768I mutations).50-52
More clinicians are requesting EGFR mutation tests for their patients with NSCLC during initial diagnosis because of improved detection techniques, availability of clinical guidelines and working group recommendations, and the increased number of testing facilities.53-58
Exons 19 and 21
Several studies have demonstrated that EGFR is uniquely expressed in tumour tissues, particularly NSCLC.13,14 Increasing use of TKI in patients with EGFR-mutated NSCLC has provided accumulating evidence that exon 19 deletions and L858R mutations are associated with differing clinicopathological characteristics and predictive and prognostic values and outcomes.7,59-61 Exon 21 mutations are more common in females, never-smokers, adenocarcinomas, and low-grade tumours.7 A pooled meta-analysis of patients with advanced NSCLC found that patients with exon 19 deletion had significantly reduced risk of disease progression than those with L858R mutation, after front-line TKI. Additionally, patients with exon 19 deletion had a longer median PFS than those with L858R mutation (HR [exon 19/21]: 0.75; 95% CI: 0.65–0.85; p<0.001) (Figure 2).62 Similar findings were demonstrated in the recent FLAURA trial, a double-blind, Phase III trial in 556 patients with previously untreated, EGFR-mutated (exon 19 deletion or L858R mutation) advanced NSCLC, which compared osimertinib (a third-generation EGFR-TKI) to gefitinib or erlotinib (a first-generation EGFR-TKI). The primary endpoint was investigator-assessed PFS. At baseline, 63% of patients had an exon 19 deletion. The investigator-assessed PFS were 21.4 months (95% CI: 16.5–24.3) and 14.4 months (95% CI: 11.1–18.9) for exon 19 deletion and L858R mutation, respectively.32 Dr Gil-Bazo believes that this difference in PFS may be because of differing tumour biology (exon 21 tumours are less angiogenic), more efficient inhibition by EGFR-TKI, and less resistance and hypersensitivity to reversible EGFR-TKI.7,62
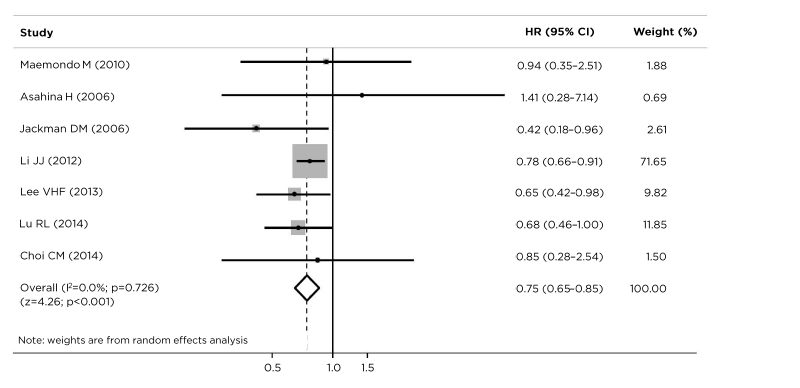
Figure 2: Progression-free survival with tyrosine kinase inhibitor therapy in patients with EGFR exon 19 deletions versus L858R mutations.
Based on the data from seven studies, patients with advanced non-small cell lung cancer with exon 19 deletion benefited from longer progression-free survival than those with an L858R mutation (HR [exon 19/21] : 0.75; 95% CI: 0.65–0.85; p<0.001).
CI: confidence interval; EGFR: epidermal growth factor receptor; HR: hazard ratio.
Data from Zhang et al.62
Benefit with Ramucirumab Regardless of Mutation Type
In the RELAY trial, 54% of enrolled patients had an exon 19 deletion and 45% had an L858R mutation. Most notably, ramucirumab plus erlotinib provided PFS benefit across most of the subgroups, with patients with exon 19 deletions and L858R mutations deriving similar benefits (19.6 months versus 19.4 months; HR: 0.651 and 0.618, respectively).9 Dr Gil Bazo evaluated the findings: “The PFS benefit with ramucirumab versus placebo was similar regardless of mutation type: exon 19 deletion (19.6 versus 12.5 months) and L858R mutations (19.4 versus 11.2 months).”
Dr Gil-Bazo explained he felt that the combined blockage of the VEGFR and EGFR pathways with ramucirumab plus erlotinib may delay the emergence of resistance to EGFR-directed therapy. The clinical implications of this trial demonstrate that combination therapy with an antiangiogenic agent and an EGFR inhibitor should be considered in the first-line setting in patients with EGFR-mutated advanced NSCLC.9 “The clinical implication of the RELAY study is that ramucirumab should be considered in the first-line setting in patients with EGFR-mutated advanced NSCLC,” he concluded.
WHAT IS THE BEST SEQUENCE OF TREATMENT FOR EGFR-MUTATED ADVANCED NSCLC?
Professor Marina Garassino
Personalised Treatment Plans
Historically, standard care prescribed to patients with cancer was without any tailored selection, with the exception of the primary site and histology of the tumour; patients with NSCLC were exclusively treated with chemotherapy without selection for histology or any other biomarker, such as EGFR mutation.21,22 The era of precision medicine has revolutionised cancer care by enabling selection of individual treatment based on specific biomarkers to reduce toxicity risk and improve treatment benefits. The therapeutic armamentarium for NSCLC has expanded significantly with the emergence of multiple targeted therapies. NSCLC was once a ‘limited-survival disease’, but now many patients are living longer because of personalised, targeted therapies.63
To match a patient’s cancer to the best treatment, clinicians need to understand the molecular profile. The use of EGFR-TKI is, therefore, based on the detection of EGFR mutations, detected via solid tissue or liquid biopsies.64 Clinical standards and testing are rapidly evolving, but based on available evidence, all patients with advanced NSCLC should be tested for EGFR mutations.53
Clinicians now need to consider several EGFR-TKI and combination strategies for the treatment of EGFR-mutated NSCLC. Randomised trials have demonstrated that second- and third-generation TKI confer significantly improved PFS versus first-generation TKI.28-32 Unfortunately, in the absence of direct head-to-head studies, it remains unclear which is the best treatment strategy for front-line use and for sequencing of agents to maximise survival benefit. Furthermore, despite initial clinical response to EGFR-TKI, acquired resistance and disease progression is inevitable at some point during treatment with EGFR-TKI.
Dual blocking of EGFR and VEGF pathways, with angiogenesis inhibition by neutralising antibodies such as ramucirumab with an EGFR-TKI, may be beneficial for improving treatment outcomes.9,43
Clinicians also have an important role in improving the patient experience with patient-centred communication and shared decision making, taking the time to inform, actively listen, understand needs and preferences, and assess a patient’s understanding of treatment options. The following factors should be considered for personalised treatment: age, comorbidities, concurrent medications, patient preference for oral medications, contraindications, presence of exon 19 deletion mutation, or presence of resistance mutation T790M.
These outcomes led Prof Garassino to advocate for an alternative standard of care with ramucirumab plus erlotinib as first-line therapy in appropriate patients with EGFR-mutated NSCLC (Figure 3).65 This would potentially enable a longer survival benefit by delaying subsequent osimertinib and chemotherapy treatment.45
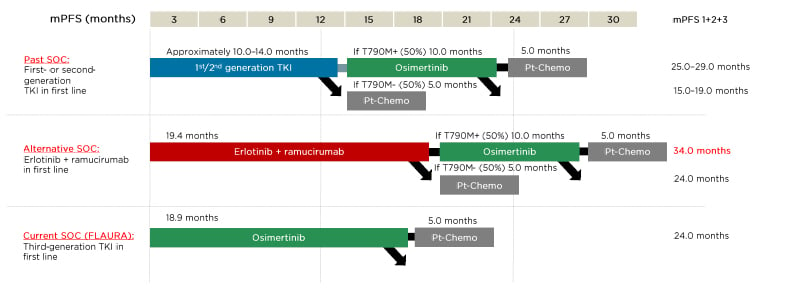
Figure 3: Potential sequencing options for patients with EGFR-mutated advanced non-small cell lung cancer.
mPFS: median progression-free survival; Pt-Chemo: platinum chemotherapy; SOC: standard of care; TKI: tyrosine
kinase inhibitor.
Adapted from Pérol.65
This novel therapeutic approach, along with shared decision making, has expanded possibilities for patients with EGFR-mutated advanced NSCLC. “These outcomes lead me to advocate for an alternative standard of care with ramucirumab plus erlotinib as first-line therapy in appropriate patients with EGFR-mutated NSCLC. This would potentially enable a longer survival benefit, by delaying subsequent osimertinib,” Prof Garassino explained.
CONCLUSION
Patients with advanced EGFR-mutated NSCLC have an unmet need for additional first-line treatment options that provide clinically meaningful benefits, including delaying disease progression and the emergence of acquired resistance. Increasing the selection of first-line treatment options for patients with EGFR-mutated advanced NSCLC provides clinicians with greater strategic choices. These include how to use the available drugs to limit toxicity, provide the best chance of long-term PFS, potentially prolong time on targeted therapy, and delay time to chemotherapy. Furthermore, recent data suggest therapeutic additivity, if not synergy, for the concurrent use of monoclonal antibodies targeting angiogenesis with EGFR-TKI. PFS now exceeds 19 months with front-line combination ramucirumab and erlotinib therapy, and with appropriate sequencing, this approach could potentially prolong time on targeted therapy and delay chemotherapy, resulting in improved patient outcomes.

![EMJ Oncology 9 [Supplement 1] 2021 feature image](https://www.emjreviews.com/wp-content/uploads/2021/01/EMJ-Oncology-9-Supplement-1-2021-feature-image-940x563.jpg)






