Meeting Summary
This was an AstraZeneca-sponsored symposium on the evolving role of immunotherapy in extensive-stage small cell lung cancer (ES-SCLC) and unresectable Stage III non-small cell lung cancer (NSCLC), as part of the virtual 2020 International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer, hosted by Singapore.
Masahiro Tsuboi, from Japan, welcomed the speakers and summarised the objectives of the seminar. The first part of the seminar focused on the role for immuno-oncology therapy in the management of unresectable Stage III NSCLC and explored the potential strategies utilising immuno-oncology therapy in the treatment of unresectable Stage III NSCLC. The second half of the seminar evaluated the available clinical data for the use of immune checkpoint inhibitors as first-line treatment for
ES-SCLC and explored the role for emerging biomarkers for immune checkpoint inhibitors in SCLC.
Glenwood Goss, from Canada, summarised the PACIFIC study and how the outcomes of this pivotal trial have influenced current immuno-oncology therapy strategies for unresectable Stage III NSCLC. Suresh Senan, from the Netherlands, continued by discussing future treatment strategies and the evolving use of immuno-oncology therapy in unresectable Stage III NSCLC.
Myung-Ju Ahn, from South Korea, introduced the audience to the role for immuno-oncology in the treatment of ES-SCLC and how classification of ES-SCLC into subgroups based on the differential expression of four transcription factors may be used to identify patients likely to respond to existing SCLC treatments.
Finally, Charles M. Rudin, from the USA, summarised the current state of existing and emerging biomarkers in SCLC and highlighted how new potential SCLC therapy targets and novel biomarkers may be used to identify patients who are more likely to respond to treatment.
Current Strategies with Immuno-oncology Therapy for Unresectable Stage III Non-small Cell Lung Cancer
Glenwood Goss
Lung cancer is classified into NSCLC (85% of patients) and SCLC (15%). NSCLC can be further classified histologically into three main types: adenocarcinoma, large cell, and squamous cell carcinoma (World Health Organisation [WHO] classification).1,2 Stage III NSCLC is defined as locally advanced lung cancer with adverse prognostic features within the primary tumour and/or the presence of metastatic disease to the regional lymph nodes only.3
For patients with Stage III NSCLC and a good performance status the current goal of treatment is cure. The standard of care for these patients who have unresectable disease has, until recently, been concurrent chemotherapy and radiation, which provides a 5-year progression-free survival (PFS) and overall survival (OS) of 18% and 32%, respectively.4 Between 1999 and 2016, several Phase III NSCLC trials have, without success, evaluated various alternative treatment strategies, such as induction chemotherapy prior to concurrent chemoradiation therapy (cCRT), consolidation chemotherapy following cCRT, increased radiation therapy dose, maintenance immunotherapy following CRT, and cCRT plus epidermal growth factor receptor inhibitor therapy.5-12 Therefore, there remains a high unmet need for more efficacious treatment.
Recently, the PACIFIC trial, which evaluated the safety and efficacy of the programmed death-ligand 1 (PD-L1) inhibitor durvalumab following cCRT, has reported positive PFS and OS outcomes.13 The scientific rationale underpinning this success is that chemoradiation induces tumour antigen release and an adaptive immune response, but concomitantly leads to PD-L1 overexpression and immune cell evasion. However, the delivery of the PD-L1 inhibitor durvalumab blocks this overexpression. Durvalumab is a human IgG1 anti-PD-L1 monoclonal antibody that has been uniquely engineered to prevent antibody-dependent cell-mediated cytotoxicity. PD-L1 inhibition by durvalumab reverses PD-L1-mediated immune suppression, which leads to a systemic antitumour response.14,15
PACIFIC was a Phase III, randomised, double-blind, placebo-controlled, multicentre, international study of durvalumab as sequential therapy in patients with locally advanced, unresectable Stage III NSCLC.13 It included patients with unresectable Stage III NSCLC without progression after definitive platinum-based cCRT (≥2 cycles) who were 18 years or older and had a WHO Performance Status (WHO PS) score of 0 or 1. Where possible, archived pre-cCRT tumour tissue was also analysed for PD-L1 expression, but this was not essential for inclusion.13
At 1–42 days post-cCRT, 713 patients were randomised 2:1, stratified by age, sex, and smoking history, to receive either durvalumab 10 mg/kg once every 2 weeks for up to 12 months (n=476), or placebo for up to 12 months (n=237). Primary endpoints in PACIFIC were PFS by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumours (RECIST, v1.1) and OS; secondary endpoints included overall response rate per BICR, duration of response per BICR, safety and tolerability, and patient-reported outcomes.13
A 4-year OS analysis of PACIFIC (median OS: 47.5 months; 95% confidence interval [CI]: 38.4–52.6 months in the durvalumab arm; versus median OS: 29.1 months; 95% CI: 22.1–35.1 months in the placebo arm) demonstrated that the stratified hazard ratio for death was 0.71 (95% CI: 0.57–0.88) at a median follow-up of 34.2 months (range: 0.2–64.9 months [Figure 1).16 Similarly, a 4-year PFS analysis (median PFS: 17.2 months; 95% CI: 13.1–23.9 months in the durvalumab arm; versus median PFS: 5.6 months; 95% CI: 4.6–7.7 months in the placebo arm) reported a stratified hazard ratio for progression or death of 0.55 (95% CI: 0.44-0.67 [hl]Figure 2[/hl]).16 Subgroup analyses of OS and PFS in prespecified subgroups favoured durvalumab over placebo, including better OS and PFS outcomes for patients younger than 65 years, and those with Stage IIIa disease, nonsquamous tumour type, prior cisplatin therapy, and absence of EGFR mutations.16 PACIFIC reported an acceptable safety profile for durvalumab therapy, with Grade 3 or 4 adverse events reported by 30.5% of patients in the durvalumab arm and 26.1% in the placebo arm. A total of 15.4% and 9.8% of durvalumab- and placebo-treated patients, respectively, discontinued the trial because of adverse events.13
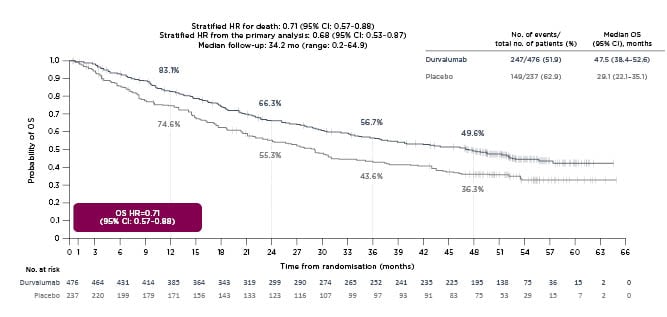
Figure 1: A 4-year analysis of PACIFIC overall survival.
Reproduced with permission from Faivre-Finn et al.16
CI: confidence interval; HR: hazard ratio; mo: month; no.: number; OS: overall survival.
The above impressive PACIFIC results have led to the recommendation that durvalumab be included in the treatment of patients with unresectable Stage III NSCLC who have not progressed after CRT in clinical guidelines such as the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines, the Pan-Asian Adapted ESMO Clinical Practice Guidelines, and The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for NSCLC.17-19
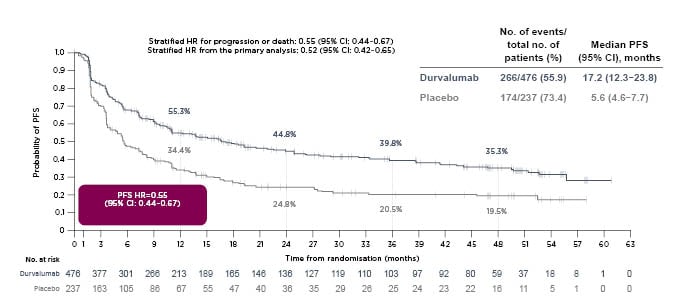
Figure 2: A 4-year analysis of PACIFIC progression-free survival.
Reproduced with permission from Faivre-Finn et al.16
CI: confidence interval; HR: hazard ratio; mo: month; no.: number; PFS: progression-free survival.
In conclusion, Goss emphasised that the success with the PACIFIC study mandates ongoing efforts to expand the role of immunotherapy and other targeted agents in combination with chemoradiotherapy in the treatment of unresectable Stage III NSCLC.
Future Strategies: Evolving Use of Immuno-oncology Therapy in Unresectable Stage III Non-small Cell Lung Cancer
Suresh Senan
After the incorporation of durvalumab following CRT into the treatment paradigm for Stage III NSCLC, several questions remain to be answered: whether sCRT can be used instead of cCRT; whether simultaneous immunotherapy is as effective as or more effective than sequential immunotherapy; and whether other or additional agents can improve upon the efficacy achieved with durvalumab. Several clinical trials are ongoing to evaluate the feasibility of using sCRT instead of cCRT, including PACIFIC-520 and PACIFIC-6.21
PACIFIC-5 is an ongoing Phase III trial comparing durvalumab versus placebo (2:1 randomisation) in patients with unresectable Stage III NSCLC without progression after definitive platinum-based sCRT or cCRT (N≈360). The primary endpoint of PACIFIC-5 is PFS by BICR and a key secondary endpoint is OS. The estimated primary completion date for PACIFIC-5 is November 2022.20
PACIFIC-6 is an ongoing Phase II trial in patients with unresectable Stage III NSCLC without progression after definitive platinum-based sCRT (N≈150). The study is evaluating treatment outcomes in durvalumab-treated patients with ECOG PS 0 or 1 (n≈120) versus durvalumab-treated patients with ECOG PS 2 (n≈30). The primary endpoint in PACIFIC-6 is incidence of Grade 3 or 4 treatment-related adverse events, and key secondary endpoints are PFS and OS. The estimated primary completion date for PACIFIC-6 is October 2021.21
Studies evaluating whether simultaneous immunotherapy is as effective as or more effective than sequential immunotherapy include PACIFIC-222 and ECOG-ACRIN 5181.23
PACIFIC-2 is an ongoing Phase III trial in patients with unresectable Stage III NSCLC, evaluating CRT plus durvalumab followed by durvalumab until progression versus CRT plus placebo followed by placebo. The primary endpoint of PACIFIC-2 is PFS, and key secondary endpoints include OS, overall response rate, duration of response, disease control rate, time to death or distant metastases, health-related quality of life, pharmacokinetics, and safety. The estimated primary completion date for PACIFIC-2 is November 2021.22
ECOG-ACRIN 5181 is an ongoing Phase III trial in patients with unresectable Stage III NSCLC, evaluating CRT plus durvalumab followed by durvalumab for 1 year versus CRT followed by durvalumab for 1 year. The primary endpoint is OS, and secondary endpoints include PFS, best objective response, and safety. The estimated primary completion date for ECOG-ACRIN 5181 is October 2028.23
Similarly, the ability of other checkpoint inhibitors to achieve similar or improved efficacy compared to that reported for durvalumab in PACIFIC is currently being investigated in several clinical trials, including the ongoing Phase II studies DETERRED (atezolizumab)24 and KEYNOTE-799 (pembrolizumab)25 and the Phase III trial CheckMate73L (nivolumab±ipilimumab).26
Although immunotherapy consolidation following cCRT has become the new standard of care for unresectable Stage III NSCLC, it has also raised multiple questions about the optimal use of immunotherapy in this setting. Questions include the optimal timing of immunotherapy and the most efficacious and tolerable agents and combinations; numerous trials are underway to explore and address these questions. Unfortunately, only a limited number of these trials (ECOG-ACRIN 5181 and CheckMate73L) include the current standard of care regimen as a comparator arm, which makes direct comparisons of these new treatment regimens with the PACIFIC regimen challenging.
The optimal dose and regimen of radiation also needs to be considered when evaluating the overall clinical benefit of immunotherapy. Senan highlighted that in PACIFIC, immunotherapy following chemo-radiotherapy led to control of known disease for a longer time, which may be a more tolerable option to increasing the radiation dosage as it may increase survival without increasing the overall toxicity that is associated with increasing radiation doses.
Defining the Role for Immuno-oncology in the Treatment of Extensive-Stage Small Cell Lung Cancer
Myung-Ju Ahn
SCLC is characterised by an exceptionally high proliferative rate, a strong predilection for early metastasis, and a poor clinical prognosis. SCLC is strongly associated with exposure to carcinogens from tobacco and most patients already have metastatic disease at the time of diagnosis, which makes curative treatment attempts challenging.27 SCLC is typically staged as either limited-stage or extensive-stage SCLC (ES-SCLC).28
Globally, approximately 355,000 new cases of SCLC are diagnosed each year and without treatment the median survival for patients with SCLC is only 2–4 months. With treatment, the 5-year survival for SCLC across all stages at diagnosis is only 6.3% versus 22.9% in NSCLC.29 SCLC was first described in 1926 and was first recognised as a neuroendocrine tumour in 1968. The first immunotherapy for SCLC was approved in 2018.29
Systemic therapies for SCLC have evolved, starting with alkylating agents introduced in the 1970s, followed by anthracycline-based chemotherapy (cyclophosphamide, doxorubicin, and vincristine) in the 1980s, platinum-based chemotherapy (etoposide–platinum [EP]) in the 1990s, and immune checkpoint inhibitor therapy in 2018.30 SCLC is a rapidly fatal disease with a 5-year survival rate that has not improved in the last 40 years. Systemic EP chemotherapy has been the standard of care for ES- or metastatic SCLC, as EP therapy has the best therapeutic index with fewer side effects compared with other treatment options. Additionally, EP therapy appears to be tolerable when combined with cCRT.31,32
Mutations in the tumour suppressor gene TP53 were first identified in SCLC in 1989. Biallelic inactivation of the tumour suppressor genes TP53 and RB1 is near universal in SCLC, and inactivating mutations in NOTCH family genes have been found in 25% of SCLC.33 As a likely consequence of the near-universal inactivation of the tumour suppressor genes TP53 and RB1, SCLC has one of the highest known tumour mutational burdens, with on average 8.9 mutations per million DNA base pairs and 7.4 protein-changing mutations per million DNA base pairs.33,34
Evidence from SCLC primary human tumours, patient-derived xenografts, cancer cell lines, and genetically engineered mouse models supports the grouping of SCLC subtypes according to the differential expression of the four key transcription regulator genes ASCL1, NEUROD1, POU2F3, and YAP1.35 The YAP1 subset of SCLC is associated with an ‘inflamed T cell’ gene expression profile and has a better prognosis and longer OS.36 Different SCLC subtypes have unique therapeutic vulnerabilities to targeted agents and chemotherapy, which may help define novel rationally targeted approaches to treat SCLC.35
Randomised clinical trials evaluating various immune checkpoint inhibitors (atezolizumab, durvalumab, nivolumab, or pembrolizumab) plus chemotherapy in ES-SCLC include the Phase III studies IMpower 133 (atezolizumab),37 CASPIAN (durvalumab),38 and KEYNOTE 604 (pembrolizumab),39 and the Phase II trials REACTION (pembrolizumab)40 and ECOG-ACRIN EA5161 (nivolumab).41 Both the IMpower 133 and CASPIAN studies demonstrated OS benefits over time with their respective immune checkpoint inhibitor combinations (median [95% CI] for IMpower 133 atezolizumab arm: 12.3 [10.8–15.8] months, control arm: 10.3 [9.3–11.3] months;42 CASPIAN durvalumab arm: 12.9 [11.3–14.7] months, control arm: 10.5 [9.3–11.2] months).43 Although the potential of characteristics such as blood-based tumour mutational burden and PD-L1 expression were evaluated as possible predictive biomarkers for immune checkpoint inhibitor therapy in ES-SCLC in both IMpower133 and CASPIAN, no correlations with improved OS were found.42,43
Even though some progress in the treatment of SCLC has been demonstrated recently for a subgroup of approximately 10% of patients with SCLC, there is still room for improvement in today’s therapeutic arsenal. Novel agents that are currently being investigated as potential novel SCLC therapies include DNA-damaging agents (lurbinectedin), vascular endothelial growth factor receptor inhibitors (apatinib, anlotinib, pazopanib, sunitinib, bevacizumab), poly-ADP ribose polymerase inhibitors (veliparib, olaparib, talazoparib), bispecific T cell engagers (BiTE, AMG 757), and immune checkpoint inhibitors with novel targets (tiragolumab).
In summary, the addition of immune checkpoint inhibitors to EP chemotherapy significantly improves OS in ES-SCLC, and atezolizumab and durvalumab have both been approved by the U.S. Food and Drug Administration (FDA) for the treatment of ES-SCLC.44,45 Ongoing clinical studies suggest that treatment with pembrolizumab and nivolumab are similar to the findings reported for atezolizumab and durvalumab. Overall, the safety outcomes for immune checkpoint inhibitors appear to be consistent with the known safety profiles of each agent, based on safety outcomes from studies in other indications. Immune checkpoint inhibitor plus chemotherapy has become the new standard of care for the first-line treatment of ES-SCLC, but given the modest survival benefit, the therapeutic response needs to be improved. Further research and clinical trials with novel agents are therefore urgently needed.
Emerging Biomarkers in Small Cell Lung Cancer
Charles M. Rudin
Approximately 10% of patients with SCLC seem to benefit from chemoimmunotherapy, either as first-line therapy (Figure 3) or as second- or third-line treatment.38,43,46-49 Rudin emphasised that even though these benefits are small, they are real. Although only 10–20% of patients may benefit, it is transformative for these patients.
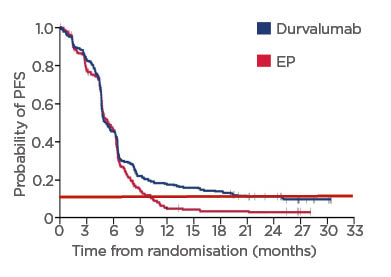
Figure 3: Proportion of patients with small cell lung cancer benefiting from first line durvalumab in CASPIAN.
Adapted from Paz-Ares et al.43
Red dotted line: proportion of patients with SCLC benefiting from long-term chemoimmunotherapy.
EP: etoposide–platinum; PFS: progression-free survival; SCLC: small cell lung cancer.
The gene SLFN11, which encodes a putative DNA/RNA helicase that is recruited to stressed replication forks and irreversibly triggers replication block and cell death, is a promising predictor of sensitivity to cytotoxic chemotherapies.50SLFN11 has emerged as a biomarker for both platinum-based chemotherapy and poly-ADP ribose polymerase inhibitor sensitivity in SCLC.51-53 As noted in the presentation by Ahn, the classification of SCLC into subtypes based on the differential expression of the four key transcription regulator genes ASCL1, NEUROD1, POU2F3, and YAP1 may be used to identify therapeutic vulnerabilities in subsets of different SCLC tumours,35 and proof-of-concept that different SCLC subtypes respond differently to treatments based on individual SCLC gene expression profiles has previously been demonstrated.54
The SCLC YAP1 subtype displays a proposed ‘T-cell inflamed signature’ gene expression signature, which includes a pattern of upregulation of many HLA genes. This ‘inflamed’ SCLC subtype has low expression of ASCL1, NEUROD1, and POU2F3, and appears to derive the greatest benefit from the addition of anti-PD-L1 therapy to chemotherapy. Interestingly, the SCLC subtypes appear to be fluid, with subtype-switching conferring resistance to platinum-based chemotherapy.55
Time will tell if gene expression subgroups or individual markers will be more useful in clinical practice, Rudin says, and this will also most likely depend on the characteristics and mechanism of action of individual drugs.
New SCLC treatment targets and corresponding biomarkers are currently being evaluated and include genes and proteins involved in diverse tumour biology processes such as cell cycle and DNA damage repair, proliferative and survival signalling, and epigenetics. Novel targeted therapy strategies aimed at those patients most likely to respond are urgently needed and are now being actively explored.27

![EMJ Oncology 10 [Supplement 4] 2022 Feature Image](https://www.emjreviews.com/wp-content/uploads/2022/02/EMJ-Oncology-10-Supplement-4-2022-Feature-Image-940x563.jpg)






