Meeting Summary
The oral vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) sunitinib is a standard first-line therapy for patients with metastatic renal cell carcinoma (mRCC).1 Survival outcomes for patients with mRCC treated with sunitinib vary between prognostic risk groups, defined by the International mRCC Database Consortium (IMDC) criteria.2,3 For example, median progression-free survival (PFS) is expected to be lower in patients with poor or intermediate-risk characteristics compared with the overall patient population, with one study reporting PFS of 5.6 months following first-line targeted therapy in patients with poor or immediate-risk characteristics compared with 7.2 months for the overall population.4 Furthermore, the presence of bone metastases is also associated with less favourable outcomes in patients with mRCC.5
Cabozantinib Versus Sunitinib (CABOSUN) as Initial Targeted Therapy for Patients with Metastatic Renal Cell Carcinoma in Poor and Intermediate-Risk Groups
One of the major challenges in treating patients with mRCC is resistance to VEGF pathway-targeted therapy. An example of such resistance occurs as a consequence of inactivation of the von Hippel-Lindau tumour suppressor gene leading to upregulation of the AXL and MET tyrosine kinases as well as VEGF. This upregulation is associated with poor prognosis and resistance to VEGFR inhibitor therapy.6-8
In an effort to overcome this challenge cabozantinib was developed and has recently been approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) as a treatment option for patients with advanced RCC who have received prior anti-angiogenic therapy (FDA) or have received prior VEGF-targeted therapy (EMA). Cabozantinib is an oral TKI whose targets include VEGFR2, MET, and AXL.9 In the Phase III METEOR study, cabozantinib met all three efficacy endpoints of improved PFS, overall survival (OS), and objective response rate (ORR) compared with everolimus in patients who had been previously treated with VEGFR TKI therapy.10,11
The cabozantinib versus sunitinib (CABOSUN) study (clinicaltrials.gov identifier: NCT01835158; sponsored by the National Cancer Institute [NCI]) is an important addition to the body of clinical knowledge regarding methods of treating mRCC because, unlike previous studies, the trial was designed to evaluate the efficacy and safety of cabozantinib versus sunitinib in patients with previously untreated mRCC, who were categorised as having poor or intermediate-risk characteristics, according to the IMDC criteria.2
CABOSUN was a randomised, multicentre, open-label, Phase II study that aimed to enrol 150 patients with advanced RCC, with an 85% power to detect a hazard ratio (HR) of 0.67 for the primary endpoint of PFS (123 events assessed by the investigators according to Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1).12 Patients were randomly allocated to one of two treatment groups and received either cabozantinib (60 mg daily, 6-week cycles) or sunitinib (50 mg daily, 4 weeks on/2 weeks off).12 Secondary endpoints included OS and ORR, which were assessed by RECIST, and safety.12 Treatment with either cabozantinib or sunitinib was continued until disease progression or intolerable toxicity resulted in treatment discontinuation.12 The efficacy analyses were performed on the intention-to-treat population, and the safety analyses undertaken only in patients who received the study drug (Figure 1).12
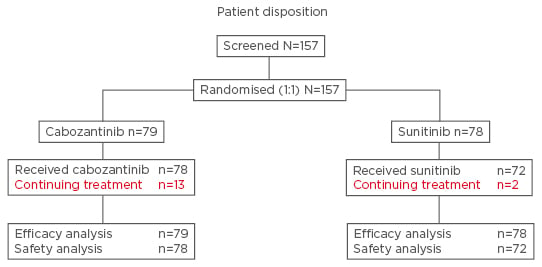
Figure 1: Patient disposition in the CABOSUN study.
Adapted from Choueiri et al. 2016.12
Baseline patient characteristics were well balanced across both treatment groups.12 As shown in Figure 2, patients treated with cabozantinib had a significantly higher PFS compared with patients treated with sunitinib (median PFS of 8.2 months for cabozantinib versus 5.6 months for sunitinib; HR adjusted for bone metastases and IMDC risk group was 0.69 [95% confidence interval (CI): 0.48–0.99]; one-sided p=0.012; data cut-off date: 15th April 2016).12 Furthermore, the significant improvement in median PFS with cabozantinib treatment was observed consistently, both in patients with poor-risk (6.3 versus 2.8 months for sunitinib; HR: 0.75, 95% CI: 0.34–1.66) and intermediate-risk (8.4 versus 6.2 months for sunitinib; HR: 0.68, 95% CI: 0.45–1.01) characteristics.12 Notably, the HR of 0.51 (95% CI: 0.29–0.90) for PFS was lower in patients with bone metastases compared with that in patients without bone metastases (HR: 0.80, 95% CI: 0.51–1.26).12 However the study was not sufficiently powered to perform statistical analyses between patient subgroups.12
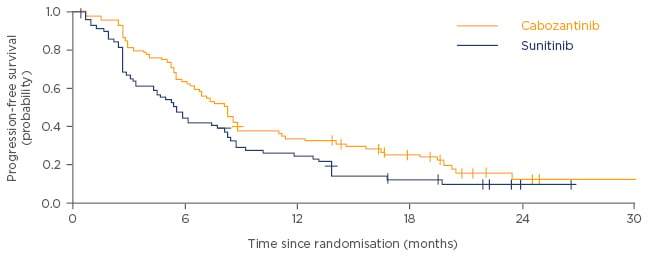
Figure 2: Progression-free survival in patients treated with cabozantinib versus sunitinib.
Adapted from Choueiri et al. 2016.12
After adjusting for bone metastases and IMDC risk group, cabozantinib was associated with a non-significant OS benefit compared with sunitinib after a median follow-up of 22.8 months (median OS of 30.3 months for cabozantinib versus 21.8 months for sunitinib; HR: 0.80, 95% CI: 0.50–1.26; Figure 3).12 Patients treated with cabozantinib also had a higher investigator-assessed ORR of 46%, compared with 18% for patients treated with sunitinib. Progressive disease was reported as the best response for 18% and 26% of patients treated with cabozantinib and sunitinib, respectively.12 Reduction in tumour target lesions was also seen in a greater proportion of patients with cabozantinib than with sunitinib (69/79 patients [87.3%] versus 34/78 patients [43.6%], respectively).12
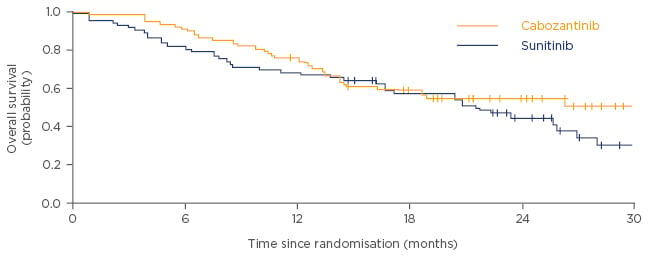
Figure 3: Overall survival in patients treated with cabozantinib versus sunitinib.
Adapted from Choueiri et al. 2016.12
The overall prevalence of all-cause adverse events (AEs), including Grade 3–4 AEs, was comparable between the two treatment arms (65% and 68% of patients for cabozantinib and sunitinib, respectively), and the safety profiles of both treatments were similar to those previously reported in patients with advanced RCC administered VEGFR inhibitors.12 The most common Grade 3–4 AEs included hypertension (28%), diarrhoea (10%), palmar plantar erythrodysesthesia (8%), fatigue (6%), increased alanine aminotransferase (5%), oral mucositis (5%), and anorexia (5%) for patients treated with cabozantinib, and hypertension (22%), fatigue (15%), diarrhoea (11%), thrombocytopenia (11%), oral mucositis (6%), neutropenia (4%), and leukopenia (3%) for patients treated with sunitinib.12 The rate of treatment discontinuation due to AEs was similar in both study arms (20% versus 21% for cabozantinib and sunitinib, respectively); although, a higher rate of dose reduction due to AEs was reported in patients treated with cabozantinib (58%) compared with patients treated with sunitinib (49%).12
Conclusion
The CABOSUN study demonstrated that cabozantinib significantly improves PFS compared with sunitinib in previously untreated patients with mRCC with poor or intermediate-risk characteristics, according to the IMDC criteria.12 Additionally, cabozantinib treatment improves ORR, with an emerging trend towards improved OS, and has a similar safety profile to that of sunitinib.12 Overall, data from the CABOSUN study indicate that cabozantinib should be a treatment option for previously untreated patients with mRCC.12








