Abstract
Endometrial carcinoma (EC) is the world’s most common gynaecological malignancy and the fourth most common carcinoma in females. Microsatellite instability high/mismatch repair deficient (MSI-H/dMMR) EC is the most represented EC subtype and is strongly associated with higher expression of anti-programmed death 1 (PD-1) receptor levels, potentially making these tumours responsive to anti-PD-1 treatment.
This case report describes the screening and treatment strategies of a 67-year-old female diagnosed with EC, which was initially treated with adjuvant therapy with chemotherapy and radiotherapy (RT). However, after the emergence of a secondary tumour lesion in the brain and other secondary body lesions, the patient started dostarlimab therapy (an anti-PD-1 monoclonal antibody) combined with RT, to evaluate a possible synergistic immune-mediated anti-tumour effect. After 5 months of therapy, the instrumental follow-up showed a complete brain lesion response, and 5 months later, a partial response of the body lesions too.
In conclusion, this case report outcome supports the combination of RT and immunotherapy to control and manage MSI-H/dMMR ECs.
Key Points
1. This article highlights the synergistic effect of the use of immunotherapy treatment and radiotherapy. The use of this combined treatment could improve the outcome of patients affected by endometrial cancer.
2. The authors highlight how the combination of radiotherapy and immunotherapy could lead to a better management of microsatellite instability high/mismatch repair deficient endometrial cancers, and how this combination could lead to a benefit even for areas distant from the irradiated site.
3. The co-operation of different physicians who collaborate in a multidisciplinary way is increasingly important for optimal management of the patient.
INTRODUCTION
Endometrial carcinoma (EC) is the world’s most common gynaecological malignancy, with a global incidence of 382,069 new cases and 89,929 deaths in 2018,1,2 expected to increase due to ageing and rising obesity.3
Post-menopausal females over 68 years are most likely to be affected by EC. Generally, most ECs are diagnosed at a localised stage, with a 5-year overall survival of 80%. In contrast, for advanced disease with lymph node invasion or metastasis, the 5-year overall survival is only 50% and 20%, respectively.2
Most patients are diagnosed at an early stage,2 so EC is generally treated with surgery with or without adjuvant therapy (radiotherapy [RT], chemotherapy) with successful results.1,2 The first-line standard treatment option for patients with advanced or recurrent EC is limited to platinum-based chemotherapy, while no second-line standard therapy is available due to patient comorbidity or performance status after first-line therapy failure.2
While conventional pathologic analysis remains crucial to EC stratification, a diagnostic algorithm using three immunohistochemical markers (p53, MSH6, and PMS2) and a molecular test (exonuclease domain mutation analysis of POLE) have also been applied to identify different prognostic groups.3
Molecularly, EC can now be classified into four prognostically relevant EC classes based on the type of mutations and somatic copy number variations, genomic characterisation, and microsatellite instability (MSI) assay, including: ultramutated cases with POLE mutations; EC cases associated with MSI status (caused by a defective DNA mismatch repair [dMMR], representing 25–30% of EC cases);2 copy number low; and copy number high.4 The POLE ultramutated and the copy number low subgroups have an excellent and good prognosis, respectively. Compared to the POLE subgroup, the prognosis is intermediate in the MSI/dMMR subgroup, while the copy number high subgroup has the worst prognosis, characterised by p53 abnormalities with a high number of somatic alterations.4 This molecular classification offers the possibility to improve the risk stratification and management of EC cases.
In MSI-H/dMMR EC, DNA replication anomalies are no longer corrected by MLH1, MSH2, MSH6, and PMS2 proteins, and this is associated with the expression of higher levels of programmed death 1 (PD-1) receptor, which potentially makes these tumours responsive to anti-PD-1 treatment.5 Indeed, among gynaecological cancers, EC highlights the greatest overexpression of PD-1 and its programmed death-ligand 1 (PD-L1).2
Dostarlimab is an anti-PD-1 monoclonal antibody that inhibits PD-1 receptor binding to PD-L1 and PD-L2, and has demonstrated durable anti-tumour activity in several tumours, including MSI-H/dMMR EC, colorectal cancer, and non-small cell lung cancer. Given its efficacy and safety profile, dostarlimab is approved as monotherapy in patients with MSI-H/ dMMR previously treated with a platinum-containing regimen.2,5
Moreover, accumulating evidence indicates that chemotherapy, RT, and other immunomodulators could synergise anti-PD-1/PD-L1 treatment by enhancing cancer antigen release, APC function, or effector activity; thus, a combined strategy is deemed as a rational and feasible approach to achieve optimal treatment effects.6
In this case report, dostarlimab therapy was suggested in a 67-year-old female diagnosed with EC to be started in combination with RT, to evaluate a synergistic immune-mediated anti-tumour effect.
METHOD
This manuscript has been prepared according to CAse REport (CARE) guidelines. Written consent was obtained from the patient to publish this case report.
CASE REPORT
At the time of presentation, due to the occurrence of metrorrhagia, a 67-year-old female underwent several diagnostic tests that led to an interventional hysteroscopy procedure at the Obstetrics and Gynecology Unit of Cannizzaro Hospital, Catania, Italy. The histological examination of a lesion tissue sample showed the presence of a widely necrotic adenocarcinoma with poor differentiation, mostly consistent with endometrioid histotype. The patient was then hospitalised, and an abdomen CT scan revealed a heterologous lesion in the uterus and nodular lesions in the left adrenal gland and the right lateroconal fascia. Centimetre- and sub-centimetre-sized lymph nodes were also observed in the interportal-caval site, lumbo-aortic site, mesenteric adipose tissue, and iliac and bilateral femoral-inguinal regions.
Two months later, a longitudinal pubo-umbilical laparotomy, total hysterectomy with bilateral adnexectomy, and systematic pelvic lymphadenectomy were performed because of the emergence of a tumour diagnosed as a poorly differentiated endometrioid adenocarcinoma (G3) of the endometrium, with a solid growth pattern and areas of necrosis (FIGO Stage IIIC G3 V1).
Based on a multidisciplinary discussion and literature guidelines,3,7 the patient was recommended to start adjuvant therapy with chemotherapy and RT, in sequence. After the second cycle of chemotherapy with carboplatin and taxol, the treatment was discontinued due to the emergence of a scalp neoformation. The lesion was surgically removed, and the histology showed a gynaecological origin. A cranial MRI showed bone infiltration and contact of the lesion with the dura mater. A CT scan revealed the presence of a secondary tumour lesion in the brain (Figure 1) and several other places.
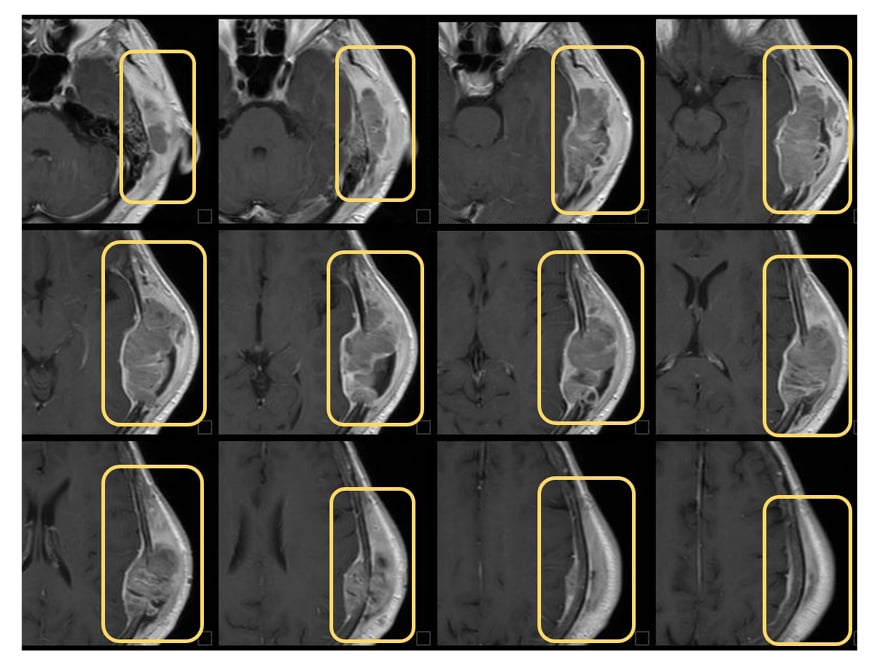
Figure 1: Cranial CT scans on brain metastasis before treatment.
Six months after initial presentation, the patient underwent RT on the left temporal-parietal lesion with a dosage of 42Gy (6Gy/Fx), and the evaluation of the MSI was initiated through two parallel methods.
The first method (PCR-MSI evaluation) required: the use of DNA extracted from both the paraffin-embedded tumour and the patient’s normal tissue; the amplification of microsatellite (STR) sequences belonging to the Bethesda panel, consisting of two mononucleotide loci (BAT-25 and BAT-26) and three dinucleotide loci (D5S346, D2S123, and D17S250); and an electrophoretic run on the QIAxcel system (Qiagen, Venlo, the Netherlands). This method showed no evidence of STR length alterations on the STRs analysed. The second method (next-generation sequencing [NGS]-MSI evaluation) consisted of testing a somatic oncology multigene panel to evaluate splicing variants, insertions and deletions, and single nucleotide variants by circulating freeDNA extraction. In addition, through liquid biopsy, 486 genes and nine loci MSI (BAT40(T)37, MONO-27(T)27, BAT26(A)27, NR24(T)23, BAT25(T)25, NR22(T)21, HSP110T17(T)17, NR21(A)21, BAT34C4(A)18) were analysed with the NextSeq 550 platform (Illumina, San Diego, California, USA). An instability emerged at the loci MONO-27(T)27, BAT26(A)27, NR24(T)23, BAT25(T)25,
NR22(T)21, and HSP110-T17(T)17, resulting in MSI-H. Other uncertain variants were also detected in some of the analysed genes, and a tumour mutational burden (TMB) with an intermediate mutational load (12.90 muts/Mb).
Subsequently, immunohistochemical analysis was performed to assess the mismatch repair (MMR) protein functional status, detecting diffuse nuclear positivity for MSH2 and MSH6 and negativity for MLH1 and PMS2, respectively, leading to the definition of unstable immunophenotype.
The patient also performed a CT scan 6 months after initial presentation, showing the presence of several body lesions (Figure 2).
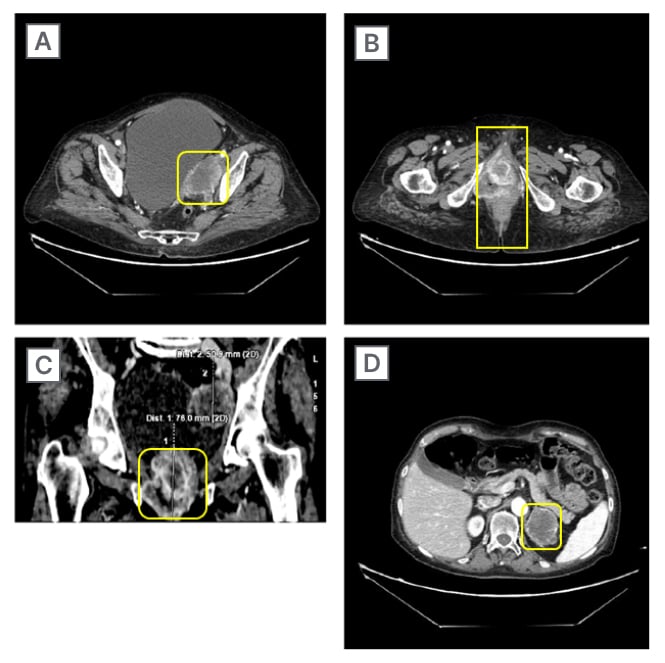
Figure 2: CT scans on different body lesions before treatment.
A) Left obturator internal iliac region; B) axial peri-anal region; C) coronal peri-anal region; and D) left adrenal region.
Around the same time, the patient (performance status: 2–3) started dostarlimab therapy at a dose of 500 mg every 3 weeks for four cycles, followed by 1,000 mg every 6 weeks for all subsequent cycles until tumour progression or severe adverse events occurrence. The instrumental follow-up scheduled 1 year after initial presentation revealed a good response pattern to treatment, with a complete brain lesion response (Figure 3). The patient was then recommended to continue the same treatment. The second follow-up, which took place 9 months after the start of dostarlimab therapy, showed a complete brain lesion response, and a partial response of the body lesions. The last follow-up performed 2 years after presentation showed an additional response to the body lesions (Figure 4).
The patient is currently on treatment, in excellent general condition, and with a performance status switched from 2–3 to 1.
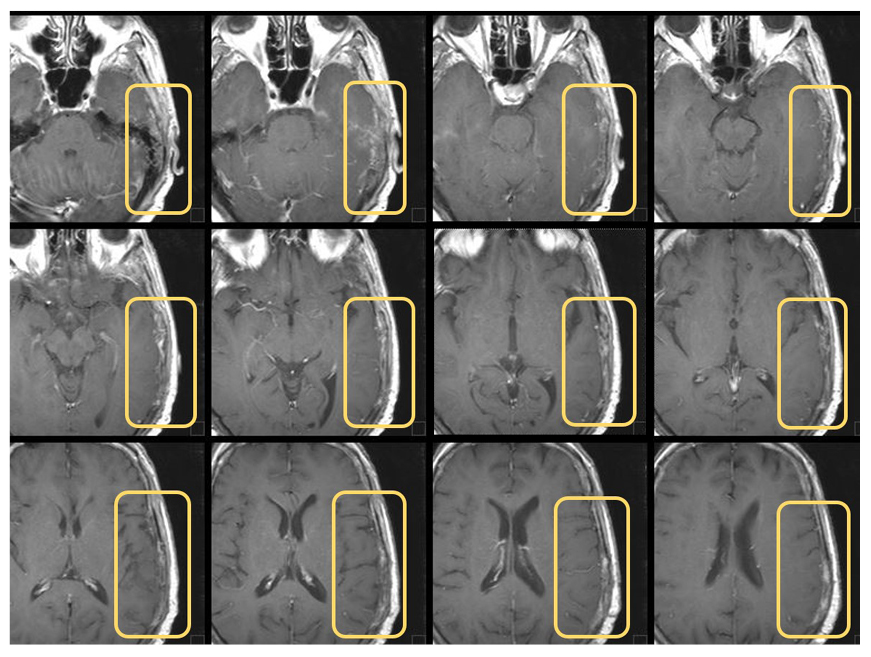
Figure 3: Cranial CT scans on brain metastasis after treatment.
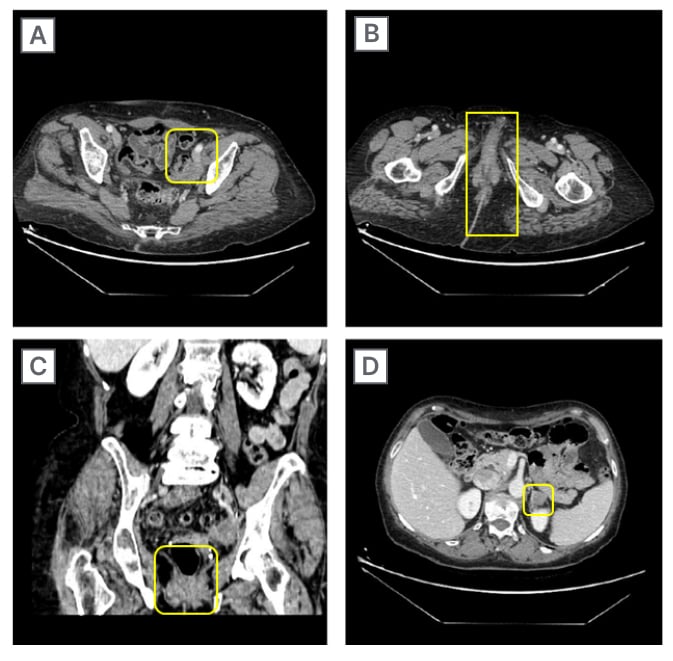
Figure 4: CT scans on different body lesions after treatment.
A) Left obturator internal iliac region; B) axial peri-anal region; C) coronal peri-anal region; and D) left adrenal region.
DISCUSSION
EC represented the fourth most common carcinoma in females in 2020, with a global incidence of 382,069 new cases and 89,929 deaths in 2018.2 Up to 30% of patients with EC are diagnosed with MSI-H/dMMR EC, in which the loss of one or more MMR proteins expression (MLH1, MSH2, MSH6, and PMS2) due to genetic or epigenetic mutations is associated with accumulated DNA replication errors at STR regions.5
As a screening strategy, this case report highlighted that performing a single test (e.g., PCR alone) when assessing MSI status can lead to false negatives. Therefore, using the latest generation and more accurate methods, such as NGS, is recommended. Several MMR/MSI assessment screening strategies are available, including PCR, immunohistochemistry, or NGS.5
Early-stage EC can be successfully treated by surgery alone, or surgery with RT or adjuvant chemotherapy (generally platinum-based chemotherapy). However, the prognosis for patients with advanced or recurrent EC is poor. To the authors’ knowledge, there are no accepted consensus-based guidelines for the standard of care after ongoing disease progression, or after a platinum-containing regimen.5
New innovative therapies are needed for advanced or recurrent ECs that do not respond to the ‘gold standard’ platinum-based chemotherapy. Among them, immunotherapy seems to be the most promising. Biomarkers effective in predicting patients who will benefit from immune checkpoint inhibitors (ICI), regardless of the tumour histology, appear to be PD-L1 expression, MSI, and TMB. In particular, it is generally accepted that a higher number of mutations (i.e., a higher TMB) is associated with an improved response of the patient’s immune system to tumour neoantigens.8
Monotherapy with ICIs, such as PD-1 inhibiting monoclonal antibodies (e.g., dostarlimab), aims to minimise the interaction of PD-1 expressed by T lymphocytes with PD-L1 and PD-L2, resulting in the constant negative feedback inhibition, enabling the immune response against cancerous cells.2 Anti-PD-1 and anti-PD-L1 antibodies have been studied in multiple haematologic and solid tumour types in primary and recurrent settings, and have been shown to be well tolerated, with a consistent safety profile.5
In addition to chemotherapy, RT has long been used to treat malignant tumours; approximately 50% of all oncologic patients have undergone RT, both for early-diagnosed and persistent or recurrent tumors.9 Typically, solid tumours respond to clinical ionising radiation through radiation-induced DNA damage, which, in turn, leads to direct tumoural cell death through cell apoptosis, senescence, and autophagy. However, these cytotoxic effects can also affect leukocytes (lymphopenia following RT), leading to RT being considered an immunosuppressive method. On the other hand, radiation-induced immune system activation has been increasingly observed in recent years, indicating that the anti-tumour response could also be elicited by RT. In addition, this immune-mediated anti-tumour effect of RT could also benefit the regression of metastasis distant from the irradiated region, and this effect is called the abscopal effect.9
This case report’s outcomes seem to be in agreement with this, as a complete response of the brain metastasis (Figure 3) and partial response of the body lesions (Figure 4) were observed 5 months after RT, performed together with the initiation of dostarlimab therapy. To date, the patient still highlights a good response to the treatment and an excellent general condition, with a performance status of 1.
Since immunotherapy can reduce patient immune tolerance toward the tumour, it is possible that combining RT and immunotherapy may amplify the anti-tumour immune response through the occurrence of the abscopal effect. As has already been previously validated, some combination therapies, including anti-PD-1/PD-L1 treatment in combination with chemotherapy, RT, and other ICIs, have greater antitumour efficacies and higher response rates compared to monotherapy.6 These combined approaches simultaneously boost multiple pathways in the cancer-immunity cycle, removing immunosuppressive inputs, and promoting an immune-supportive tumour microenvironment.
This synergistic anti-tumour effect has already been documented in pre-clinical and clinical studies,6,9-11 supporting the combination of RT and immunotherapy for TMB control and management of MSI-H/dMMR EC.
CONCLUSION
EC is the world’s most common gynaecological malignancy, with most patients with EC diagnosed with the MSI-H/dMMR EC subtype, which is strongly associated with the expression of higher PD-1 receptor levels, potentially making these tumours responsive to anti-PD-1 treatment.
In this scenario, the anti-tumour activity of dostarlimab (an anti-PD-1 monoclonal antibody) has been investigated, in combination with RT, in a 67-year-old female diagnosed with MSI-H/dMMR EC, who presented with a secondary brain tumour lesion, and several other body lesions. After 5 months of therapy, the instrumental follow-up showed a complete brain lesion response, and 5 months later, it has been possible to also evaluate a partial response of the body lesions.
The authors suggest that this anti-tumour response could also be enhanced by the immune-mediated anti-tumour effect of RT, which was performed together with the initiation of dostarlimab therapy, and also allowed the regression of metastasis distant from the irradiated region through the abscopal effect. In conclusion, this case report supports the combination of RT and immunotherapy for TMB control and management of MSI-H/dMMR EC.







