BACKGROUND
Osteosarcoma is the most common primary bone tumour in adolescents and young adults, with no survival improvement in the last few decades. Lenvatinib (LEN) is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1–3 and other targets. This study reports data from Phase Ib dose-finding and Phase II expansion cohorts of LEN with etoposide and ifosfamide chemotherapy in patients with relapsed or refractory osteosarcoma.1 Single-agent safety and efficacy data were presented at the American Society of Clinical Oncology (ASCO) meeting in 2018.2
METHODS
Patients of the study were aged 2 to ≤25 years, with relapsed or refractory osteosarcoma and <2 prior VEGF-targeted therapies. The Phase Ib starting dose of LEN was 11 mg/m2/day, ifosfamide 3000 mg/m2, and etoposide 100 mg/m2 daily for 3 days. On determination of the recommended Phase II dose (RPh2D) of LEN with chemotherapy, patients were enrolled into the Phase II expansion cohort. The primary endpoint of Phase Ib was upon RPh2D determination and Phase 2 endpoint was determined by 4 month progression-free survival (PFS-4).
RESULTS
In the Phase Ib dose-finding cohort (n=22), patients received LEN 11 mg/m2 (n=7) and 14 mg/m2 (n=15) with chemotherapy. Dose-limiting toxicities included Grade 4 thrombocytopenia (n=1; LEN 11 mg/m2), Grade 4 thrombocytopenia and Grade 3 epistaxis (n=1; LEN 14 mg/m2), Grade 2 oral dysesthesia, Grade 3 muscle spasm, and Grade 2 back pain (n=1; LEN 14 mg/m2). RPh2D was recorded as LEN 14 mg/m2 with chemotherapy. In the expansion cohort (n=20), the median number of LEN cycles received was 4 (range: 1–7).
As reported in the database, the most frequent treatment-emergent adverse events (TEAE) were decreased platelet count or thrombocytopenia (50%/30%), neutropenia or neutrophil count decrease (45%/25%), anaemia (45%), nausea (40%), alanine aminotransferase level increase, diarrhoea, and white blood cell count decrease (30% each). The most frequent Grade ≥3 TEAE were neutropenia or neutrophil count decrease (45%/25%), platelet count decrease or thrombocytopenia (40%/20%), white blood cell count decrease (30%), and anaemia (25%). Most of these side effects were chemotherapy related. Pneumothorax was observed in the dose-finding cohort (n=6) and expansion cohort (n=1); two (dose-finding cohort) were Grade ≥3, and one was post-thoracotomy. In the dose-finding cohort, four patients discontinued treatment due to TEAE. There were no fatalities reported as a result of a treatment related TEAE.
In terms of efficacy, in the dose-finding combination cohort, 12 out of 18 evaluable patients (66.7%) achieved PFS-4 (Figure 1A). In the Phase II expansion cohort, 5 out of 8 evaluable patients (62.5%) achieved PFS-4 (Figure 1B).
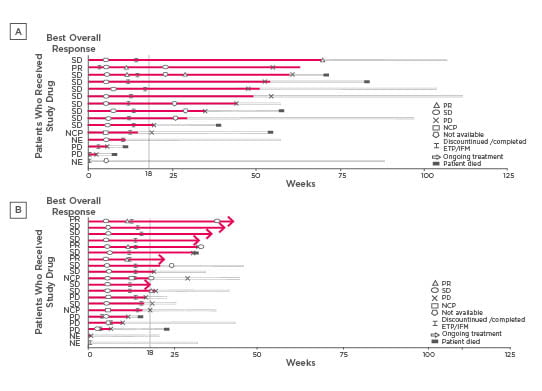
Figure 1: Duration of treatment, best overall response, and change of response over time (full analysis set: Lenvatinib [14 mg/m2] plus IFM plus ETP) for Phase Ib (A) and Phase II (B).
Each bar with solid line represents treatment duration, while the extended bars with the dashed lines represent the duration that the patient remained on the study after treatment discontinuation. A dose of 14 mg/m2 of Lenvatinib had been planned.
ETP: etoposide; IFM: ifosfamide; NCP: non-complete response or progressive disease; NE: not evaluable; PD: progressive disease; PR: partial response; SD: stable disease.
CONCLUSION
The combination of RPh2D LEN (14 mg/m2) with chemotherapy has a manageable safety profile with promising preliminary evidence of efficacy. In 2020, a randomised Phase II Trial (etoposide and ifosfamide) with or without LEN will take place (E7080-G000-230/ITCC-082) to evaluate the added value of LEN to second line chemotherapy in refractory or relapsed osteosarcoma.








