BACKGROUND
Adoptive transfer of T cell receptor (TCR)-redirected T cells has been reported to exhibit efficacy in some patients with melanoma and sarcoma.1 However, cytokine release syndrome (CRS) and its relation to tumour response has not been well-documented. This study aimed to evaluate clinical responses in association with the cell kinetics and CRS after transfer of high-affinity NY-ESO-1 TCR-gene transduced T cells in cancer patients.2
METHODS
The authors developed a novel-type affinity-enhanced NY-ESO-1-specific TCR and an originally-developed retrovirus vector that encodes small interfering RNA (siRNA) to silence endogenous TCR creation.3 The NY-ESO-1/TCR sequence was mutated for high affinity with replacements of G50A and A51E in the CDR2 region.4 This was a first-in-human clinical trial of the novel NY-ESO-1-specfic TCR-T cell transfer to evaluate the safety, in vivo cell kinetics, and clinical responses. It was designed as a cell-dose escalation from 5×108 (cohort 1) to 5×109 (cohort 2) cells. NY-ESO-1-expressing refractory cancer patients were enrolled with a 3+3 cohort design. Eligibility criteria included being ≥20 years of age, recurrent/refractory tumour, NY-ESO-1 positive expression in the tumour specimen, HLA-A*02:01 or *02:06 (+) for NY-ESO-1, and informed consent. A 200 mL blood draw from each patient was obtained. TCR-gene transduction and culture were carried out for 10–12 days, followed by deep freezing quality check. Cyclophosphamide (1,500 mg/m2) was administered prior to the TCR-T cell transfer as preconditioning.
RESULTS
Nine patients were treated with the TCR-T cells that expanded in peripheral blood with a dose-dependent manner, associated with rapid proliferation within 5 days of infusion. Three patients receiving 5×109 cells developed early-onset CRS, with elevated levels of serum IL-6 and IFN-γ. These CRS on Day 1 or 2 were well managed with tocilizumab treatment. Three synovial sarcoma patients exhibited tumour shrinkage and partial responses, and they all had high-expression of NY-ESO-1 in the tumour samples, namely 75% or more (Table 1). Intriguingly, tumour regrowth seemed to be inversely correlated with a steep decrease in the number of the TCR-gene-transduced lymphocytes after the TCR-T therapy, especially in the patient with high tumour burden, such as TBI1301-16. Exploratory analysis revealed that multiple chemotactic cytokines, including CCL2 and CCL7, as well as IL-3 increased in the serum of patients with CRS. The proportions of effector-memory phenotype T cells in the infused cell-product were significantly associated with CRS development.
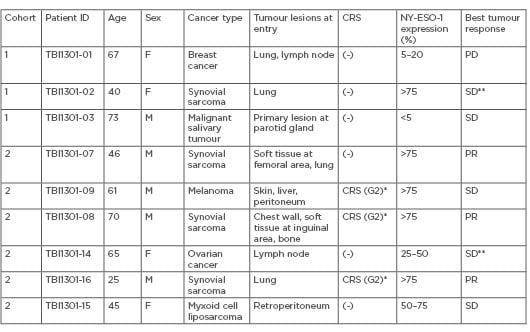
Table 1: Patients’ characteristics who received adoptive transfer of TBI-1301.
*Tocilizumab was used to treat CRS. **Cases without measurable lesions.
CRS: cytokine release syndrome; PD: progressive disease; PR: partial response; SD: stable disease.
CONCLUSION
The affinity-enhanced NY-ESO-1/TCR-T cell transfer exhibited early-onset CRS in association with in vivo cell proliferation and sequential tumour responses in the patients with high-NY-ESO-1-expressing synovial sarcoma. Further understanding and development of the TCR-T therapies are needed to increase the ability to overcome the challenges of treating solid tumours, such as high tumour burden patients.








