Meeting Summary
Parkinson’s disease (PD) is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. There are currently limited treatment options, including levodopa (L-DOPA), which can be amended in dosing (e.g. schedule and strength), alternative L-DOPA formulations (e.g. quick-acting soluble formulations, extended-release formulations, and continuous infusion), as well as enzyme inhibition (catechol-O-methyl transferase [COMT] and monoamine oxidase B [MAO-B] inhibitors), dopamine agonists (DAs), and combinations thereof. Besides treating symptoms, one of the main concerns in PD is to strike a fine balance between treatment being efficacious without causing dyskinesia, and treatment ‘wearing off’ due to short therapy half-life.
Conventional COMT inhibitors, entacapone and tolcapone, have shown promising results in reducing L-DOPA fluctuations and improving motor function; however, the novel once-daily (OD) oral COMT inhibitor opicapone has an exceptionally high binding affinity with the COMT enzyme, translated into a long duration of action, and provided consistent L-DOPA fluctuation control over 24 hours. Opicapone treatment is associated with more efficient endogenous L-DOPA utilisation and less need for exogenous L-DOPA. The long-term benefits of opicapone have been demonstrated in patients initiated on opicapone and those switching from combination treatment with entacapone. The reported reductions in ‘off-time’, a state of decreased mobility, and favourable results for dyskinesia, may have a big impact on patients’ mobility and treatment adherence; however, further assessments are required.
Levodopa-Related ‘Wearing Off’ in Parkinson’s Disease: Current Therapeutic Options
Professor Werner Poewe
Motor fluctuations are significant complications of L-DOPA therapy that affect many patients with advancing PD. A pragmatic and internationally accepted definition of motor fluctuations, also used in clinical trials, is the description of the ‘wearing off’ phenomenon, stated as: ‘A generally predictable recurrence of motor or non-motor symptoms that precedes a scheduled dose and usually improves with anti-parkinsonian medication’.1 ‘Wearing off’ of treatment is highly related to L-DOPA availability. Concomitant medications that potentially lead to pharmacokinetic changes of L-DOPA or prolong striatal dopamine stimulation should be considered when deciding treatment suitability for individual patients.
There are multiple ways in which L-DOPA-related fluctuations of motor function can arise in patients with PD (Figure 1). Owing to a half-life of ~90 minutes, fluctuations at plasma level can affect dopamine concentrations at the synaptic level and potentially reduce storage capacity at the presynaptic level, leading to a ‘wearing off’ of therapeutic L-DOPA. Another mechanism, termed ‘delayed on’, is associated with a delay in gastric emptying or difficulty with intestinal absorption, which may result in a delayed uptake and subsequent effect of therapeutic L-DOPA. The ‘delayed on’ mechanism mainly occurs in the early morning, causing severe motor problems. A phenomenon termed ‘no-ON’ describes dose failure most often caused by delayed gastric emptying, problems with intestinal absorption related to competition between food-derived amino acids and L-DOPA, or problems in the blood–brain barrier transport of L-DOPA. Potential changes in striatal pharmacodynamics or a change in patient perception of the severity of their disease symptoms may lead to ‘random ON-OFF’ fluctuations, which are currently poorly understood.
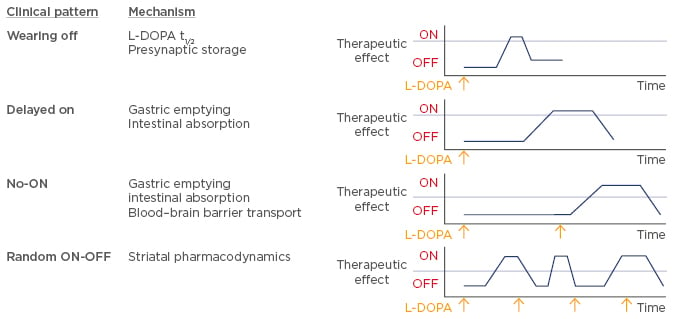
Figure 1: Classification of L-DOPA-related fluctuations of motor function.
L-DOPA: levodopa.
There is a high prevalence of motor complications in patients treated with L-DOPA. Early studies indicated that a high percentage of patients developed motor fluctuations within 5–6 years after L-DOPA treatment initiation,2-5 although it needs to be highlighted that patients in these studies received high therapeutic doses of L-DOPA. More recent investigations led to the conclusion that patients’ age, duration of the disease, and treatment dose of L-DOPA are risk factors for motor fluctuations. In particular, there is an increased propensity of young patients to develop motor complications and dyskinesia early during the disease.
The prevalence of motor fluctuations is surprisingly high even after short disease duration.6-8 In two short-term studies, the ELLDOPA study6 and the DATATOP study,9 30% and 50% of patients with PD, respectively, reported ‘wearing off’, when explicitly asked, therefore, ‘wearing off’ should be discussed with patients during the early stages of treatment. In a survey of 173 patients with a disease duration of >6 years,10 fluctuating response to medication was named as the most problematic and severe symptom in advanced PD, closely followed by mood changes, drooling, and sleep problems, demonstrating the need for more effective therapies.
Conventional Modifications of Levodopa
Modifications of L-DOPA range from simple to more complicated approaches, some of which are still under investigation. Conventional pharmacotherapy includes changes in dosing (e.g. schedule and strength), alternative L-DOPA formulations (e.g. quick-acting soluble formulations, extended-release formulations, and continuous infusion), as well as enzyme inhibition (COMT and MAO-B inhibitors) and DAs.
Shortening of L-DOPA dosing intervals by increasing the number of administrations is a method currently used in clinical practice to prevent ‘wearing off’. However, with a switch in the number of doses per day, treatment adherence becomes more problematic. It has also been shown that higher doses increase the ‘on-time’ per L-DOPA dose, but at the same time, raise the possibility of developing dyskinesia.
Soluble L-DOPA tablets have been developed to shorten the time to effect, which has proved useful in counteracting the early morning ‘delayed on’ phenomenon. Varying success has been reported with extended-release tablets that were developed to increase the half-life and bioavailability of L-DOPA. Although very popular and slightly extending exposure to L-DOPA, clinical studies did not convincingly report significant increase in ‘on-time’ and symptom relief over prolonged periods with extended-release tablets. New extended-release treatment options are currently being developed, with IPX066 now marketed in the USA and approved in Europe. IPX066 reportedly increases L-DOPA plasma levels compared with a standard L-DOPA formulation and a maximum concentration (Cmax) >50% after 4 hours compared with 3 hours, respectively.11 However, as is often seen with extended-release L-DOPA formulations, IPX066 was associated with decreased L-DOPA bioavailability, resulting in the need for higher dosing.
COMT inhibitors play an important role in modifying the pharmacokinetics of L-DOPA. Two COMT inhibitors are currently available and treatment guidelines recommend entacapone as first-line and tolcapone, although slightly more potent,12 as second-line treatment owing to liver monitoring requirements. The net effect with respect to ‘off-time’ reduction for entacapone has been reported to be 41 minutes.13 Both COMT inhibitors improve motor fluctuations in the ‘on’ stage14 and are associated with a cumulative effect when combined with MAO-B inhibitors, increasing L-DOPA availability peripherally with entacapone and centrally with MAO-B.15
Three MAO-B inhibitors are currently available; selegiline, rasagiline, and safinamide, the latter being the only reversible MAO-B inhibitor with a half-life of 20–30 hours.16-18 Clinical evidence shows benefits in motor fluctuations with all three MAO-B inhibitors. In two large Phase III trials in patients experiencing motor fluctuations, rasagiline was associated with a net reduction in ‘off-time’ by approximately 47–60 minutes.19,20 Similar results were observed with safinamide in the SETTLE study.21,22
DAs also play an important role in providing treatment options for ‘wearing off’ owing to a much longer half-life compared with L-DOPA.23
However, their low effectiveness in treating Parkinson’s symptoms results in the need for combination therapy with L-DOPA. The efficacy of DAs has been intensely investigated, and most trials confirmed a net effect of 1–2 hours,24-34 which is slightly superior to that of currently available COMT and MAO-B inhibitors (Table 1).
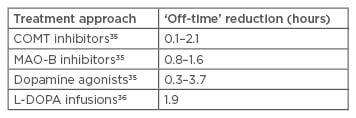
Table 1: Off-time reduction of different Parkinson’s treatment approaches.
COMT: catechol-O-methyl transferase; L-DOPA: levodopa; MAO-B: monoamine oxidase B.
The non-ergoline DA apomorphine is the only DA with comparable efficacy with L-DOPA in preventing ‘wearing off’. It has a short half-life of 40–60 minutes, and subcutaneous administration results in a short latency, well suited to quickly interrupt the ‘off’ phase. As ‘wearing off’ is measured not only by summation of ‘off-time’ throughout a patient’s day, but also time to treatment effect, subcutaneous apomorphine injections, soluble L-DOPA tablets, and inhalation approaches currently in development, provide the quickest relief.
Escalated Modifications of Levodopa
During the progression of PD, conventional therapies and combination thereof may not yield the same efficacy results as during early stages of the disease; thus, escalated therapies based on dopamine pumps and deep brain stimulation (DBS) may be required.
As well as being administered subcutaneously, apomorphine can also be administered via continuous subcutaneous infusion using a relatively small pump system. Pump systems have been used since the 1980s and show dramatic reductions in ‘off-time’. Recently small-scale, long-term, observational studies reported impressive effects with continuous subcutaneous infusion of apomorphine in ‘off-time’ reduction and improvements in dyskinesia,37-44 which were replicated in a randomised trial showing 4–5 hours more spent ‘on-time’ with L-DOPA.36
DBS is currently the last-in-line treatment option after continuous infusion and has been associated with ‘off-time’ reductions >60%;45 however, discussions around the timing of DBS initiation are ongoing.
Levodopa-related ‘Wearing Off’ in Parkinson’s Disease: New Developments
Professor Georg Ebersbach
Currently ongoing or recently completed trials have been investigating novel therapies such as parenteral dopaminergic treatment (Phase II), adenosine A2A receptor antagonists (Phase III), extended-release amantadine, and IPX066 (both Phase III), as well as the COMT inhibitor opicapone, which has now been approved by the European Medicines Agency (EMA).
Novel Parenteral Dopaminergic Therapy
There are three new options for parenteral dopaminergic therapy. Subcutaneous L-DOPA infusions provide a less invasive and risk-prone approach compared with intrajejunal infusion,46 a novel pH-neutral apomorphine formulation may be associated with fewer skin irritations,47 and finally, apomorphine inhalation may provide a novel way of administration and has shown promising results in ‘off-time’ reductions compared with placebo.48
Adenosine A2A Receptor Antagonists
Adenosine A2A receptor blockade inhibits the hyperactive GABAergic striato-pallido projection and leads to activation of the (inhibitory) pallido-subthalamic transmission. Istradefyllin and tozadenant are at the Phase III trial stage, with the former reporting moderate ‘off-time’ reduction.49
Extended-Release Amantadine and IPX066
As previously mentioned, IPX066 significantly reduced the ‘off-time’ period compared with standard L-DOPA.50 Amantadine has been used in clinical practice for many years, and extended-release amantadine reflected previous benefits regarding dyskinesia. Additionally, an ‘anti-off-effect’ was observed with a reduction in ‘wearing off’, indicating that the anti-dyskinetic effect is accompanied by pro-kinetic effects.51
Opicapone: A New COMT Inhibitor
Opicapone is characterised by an exceptionally high binding affinity with the COMT enzyme and strong inhibition over 24 hours,52,53 making it suitable for OD intake and allowing prediction of overnight treatment cover. While both opicapone and entacapone are active peripherally, entacapone is associated with stronger L-DOPA fluctuations, making it more difficult to dose and monitor. Opicapone’s pharmacokinetic profile of strong binding with COMT translates into higher bioavailability of L-DOPA (24% and 55% higher compared with entacapone and placebo, respectively)54 and facilitates a simplified regimen that tailors existing L-DOPA to maximise its clinical benefit.
Two pivotal Phase III trials, BIPARK I55 and BIPARK II,56,57 investigated opicapone compared with entacapone and placebo, respectively. The BIPARK I study included five treatment arms: placebo, entacapone 200 mg, and opicapone 5, 25, and 50 mg. Following 3 weeks of treatment adjustment, the trial duration was 12 weeks with a 2-week follow -up and a 1-year open-label extension. Patients were 65 years of age and diagnosed 7–7.5 years prior, with a mean ‘off-time’ of 6–7 hours/day. Treatment with opicapone 50 mg led to an absolute 2-hour reduction in ‘off-time’. Numerically, reduction of ‘off-time’ was greater with opicapone than entacapone (-1.6-hour reduction). Non-inferiority versus entacapone was confirmed. Responders to treatment, defined as patients achieving ≥1-hour reduction in ‘off-time’, were statistically significantly higher with opicapone 25 mg (p<0.05) and 50 mg (p<0.01) versus placebo, while numerically more patients (~70%) responded to opicapone 50 mg, compared with entacapone (~58%; not significant versus placebo). High-dose opicapone reported the longest ‘on-time’ duration, without troublesome dyskinesia; however, this was not significantly different between active treatment groups. Clinical results were reflected in subject/investigator-reported outcomes (subject’s and investigator’s global assessment of change: SGAC and IGAC) with >70% of investigators rating the patients’ condition as improved compared with the beginning of the trial, which was statistically significantly higher for the opicapone 50 mg group compared with placebo (p=0.0008) and entacapone (p=0.0091) (Figure 2). No unexpected adverse events were reported and the increased rate of dyskinesia in the opicapone groups was likely the result of higher L-DOPA bioavailability. However, dyskinesia rates should not be of concern, as L-DOPA dosing can be adjusted in clinical practice, which was not the case in this trial. Opicapone OD is well tolerated and gastrointestinal tolerability (diarrhoea and nausea), usually a concern with tolcapone and entacapone, and hepatic adverse events were not reported with any opicapone dose, thus liver monitoring is not required.
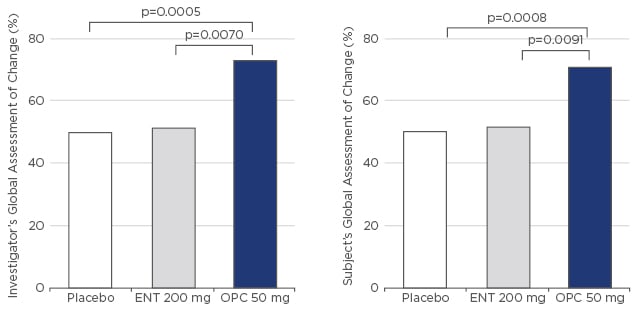
Figure 2: BIPARK I – Global assessment of change.
ENT: entacapone; OPC: opicapone.
Outcomes reported for BIPARK I were supported by BIPARK II outcomes, based on comparison with placebo only, adjustment of L-DOPA dose, and a broader global patient population. BIPARK II assessed the efficacy and safety of opicapone in idiopathic PD patients with ‘wearing off’ phenomenon treated with L-DOPA plus a dopa decarboxylase inhibitor (DDCI). During the open-label extension phase, patients switched from placebo or entacapone benefitted from a further statistically significant reduction in ‘off-time’ (0.7 hours) and significant increases in ‘on-time’.
Overall, opicapone demonstrated increased L-DOPA bioavailability, increased ‘on-time’ and reduced ‘off-time’ in a OD administration, providing a clear enhancement of the current treatment options with COMT inhibitors.
Levodopa-related ‘Wearing off’ in Parkinson’s Disease: Clinical Practice
Professor Thomas Müller
L-DOPA has a relatively short half-life of ~90 min, which causes concentration fluctuations in peripheral plasma and dopamine in the presynaptic cleft after crossing the blood–brain barrier. These fluctuations lead to non-physiological stimulation of postsynaptic receptors, resulting in motor complications like ‘wearing off’ or dyskinesia, which could be reduced with adequate medication, supporting a more physiological stimulation in the striatal circuitry. Between-patient variability in L-DOPA resorption complicates treatment algorithms, and administration frequency and dosing are adjusted throughout each day to carefully balance the complex interplay between ‘off-time’ and dyskinesia. However, these adjustments may have an impact on patient adherence to treatment and may potentially increase the risk for dyskinesia, as was demonstrated in the ELLDOPA dose-ranging study, where L-DOPA-naïve patients had an increased risk of motor complications due to ‘wearing off’ and dyskinesia with increasing doses of L-DOPA (30% and 17%, respectively, with 600 mg/day).6
Current Treatment Algorithms in Clinical Practice
Current clinical practice for the treatment of PD is based on L-DOPA, potentially in combination with COMT inhibitors entacapone or tolcapone. The STRIDE-PD study investigated the potential of delaying the onset of motor complications and improvement of motor control and quality of life with combination treatment versus individual compounds.58 Although the study did not meet its primary endpoint (likely due to stringent treatment adjustment criteria), careful evaluation of the study data indicated a trend towards prediction of development of motor complications based on the cumulative amount of L-DOPA administered per day, and patient weight and age as additional risk factors. In line with previous findings,59,60 lower weight seems to correlate with higher fluctuations of L-DOPA plasma levels,58 likely owing to the lack of muscle tissue to absorb L-DOPA.
It has been hypothesised that extended-release L-DOPA and/or COMT inhibition can reduce fluctuations of L-DOPA plasma levels, resulting in more uniform availability of L-DOPA in the brain. A small-scale study investigated the effect of treatment changes from twice-daily administration of: L-DOPA 100 mg/carbidopa in Week 1, L-DOPA 100 mg/carbidopa/entacapone in Week 2, and L-DOPA 100 mg/carbidopa/tolcapone in Week 3.61 Pharmacokinetic analysis demonstrated no significant differences between treatment combinations, suggesting similar L-DOPA bioavailability for all treatment regimens. However, further analysis showed significantly fewer reductions in L-DOPA plasma levels, significantly reduced fluctuation in L-DOPA plasma levels and significantly improved fine motor skills with entacapone and tolcapone, compared with L-DOPA/carbidopa, indicating more constant availability of L-DOPA.61
Overall, this study highlighted the importance of the role of COMT inhibitors in improving motor function by regulating L-DOPA plasma levels, fluctuations of L-DOPA plasma levels, resorption of L-DOPA, L-DOPA metabolism, and inhibition of enzymes metabolising L-DOPA.62
A Novel COMT Inhibitor: Opicapone
Compared with entacapone, where strong L-DOPA fluctuations were reported for three doses over 24 hours, the novel OD COMT inhibitor opicapone significantly and constantly inhibited COMT activity over several days,53 which shows that COMT inhibition is a constant factor in the pharmacokinetics of L-DOPA and allows treating physicians to more reliably predict COMT inhibition and significantly improve L-DOPA fluctuation (Figure 3).55,56
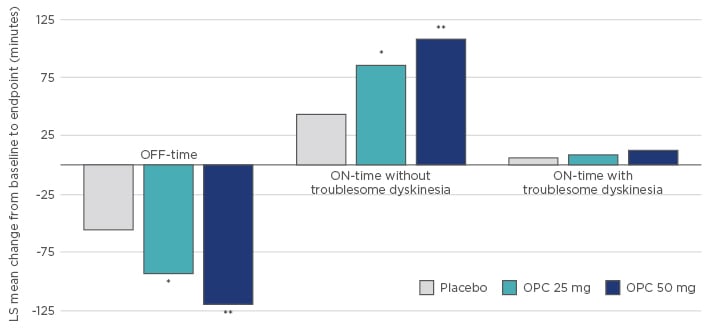
Figure 3: Effect of opicapone on motor function in patients with Parkinson’s disease.
*p<0.05, **p<0.0001 versus Placebo.
LS: least square; OPC: opicapone.
Opicapone Use in Clinical Practice
In general, treatment changes should only be made if a patient is dissatisfied with their current treatment. Patients experiencing classical L-DOPA/carbidopa treatment ‘wearing off’ would be suited for treatment with opicapone, where one additional tablet administered OD in the evening might significantly reduce their ‘off-time’. Development of dyskinesia in these patients initiated on opicapone is usually a sign of treatment effect and should be balanced by reducing L-DOPA dosing by 25–50 mg per day or as relevant to the individual patient. When amending treatment, it is not recommended to combine two COMT inhibitors. Instead, a treatment switch from combination of L-DOPA/carbidopa/entacapone to L-DOPA/carbidopa plus opicapone is recommended by administering regular doses of L-DOPA/carbidopa with an additional evening dose of oral opicapone.
When thinking on the switch from entacapone to opicapone, patients should be closely monitored for development of dyskinesia; however, patients switching to opicapone will not show signs of urine discoloration, as would be the case with entacapone. On the other hand, patients on opicapone have no need for liver monitoring, as is the case with tolcapone.
Conclusions
Fluctuations of L-DOPA plasma levels are one of the main reasons for development of motor fluctuations. COMT inhibition supports the effect of L-DOPA by providing more constant L-DOPA plasma levels, thereby reducing motor complications. The novel COMT inhibitor opicapone is an OD oral treatment with more continuous COMT inhibition compared with entacapone and tolcapone, thus greatly simplifying L-DOPA therapy.








