Chair person: Bastiaan R. Bloem1
Speakers: Werner Poewe,² Georg Ebersbach³
1. Radboud University Medical Center, Department of Neurology, Nijmegen, the Netherlands
2. Medical University Innsbruck, Department of Neurology, Innsbruck, Austria
3. Movement Disorders Clinic, Kliniken Beelitz, Beelitz, Germany
Disclosure: Prof Bloem has received consultancy fees from AbbVie, Biogen, UCB, and Walk with Path; has received grants/research support from AbbVie, Gatsby Foundation, Horizon 2020, The Michael J. Fox Foundation, Netherlands Organisation for Scientific Research, Parkinson Vereniging, Parkinson’s Foundation, Topsector Life Sciences & Health, Stichting ParkinsonFonds, UCB, and Verily Life Sciences; and has received fees for speaking at conferences from AbbVie, Bial, Roche, and Zambon Pharma. Prof Poewe has received personal fees from AbbVie, AFFiRiS, AstraZeneca, Bial, Britannia Pharmaceuticals, Denali Therapeutics, Intec, Lundbeck, Neurocrine Biosciences, Neuroderm, Novartis, Orion Pharma, Takeda, Teva, UCB, and Zambon Pharma (consultancy and lecture fees in relation to clinical drug development programmes for Parkinson’s disease); has received royalties from Cambridge University Press, Oxford University Press, Thieme, and Wiley Blackwell; and has received grant support from EU FP7, Horizon 2020, and The Michael J. Fox Foundation. Prof Ebersbach has received honoraria for advisory boards from AbbVie, Bial, Desitin Arzneimittel GmbH, Neuroderm, and STADA Arzneimittel AG; has received honoraria for speaking engagements from AbbVie, Bial, Britannia Pharmaceuticals, Desitin Arzneimittel GmbH, Licher GmbH, UCB, and Zambon Pharma; and has received royalties from Kohlhammer Verlag and Thieme Verlag.
Acknowledgements: This article was developed with writing and editorial support from Makara Health Communications and funding support from Bial.
Support: The symposium and publication of this article were funded by Bial.
Citation: EMJ Neurol. 2020;8[1]:28-37.
Meeting Summary:
After five decades, levodopa is still considered the gold standard for the treatment of motor symptoms in patients with Parkinson’s disease (PD). However, after the initial years of levodopa therapy many patients develop motor response oscillations such as end-of-dose wearing-off and levodopa-induced dyskinesias. The mechanisms underlying the development of levodopa response fluctuations are multifactorial, but perhaps the most important factor contributing to the end-of-dose wearing-off phenomenon is levodopa’s short half-life. Limitations in the bioavailability of levodopa have led to dopa-decarboxylase inhibitor (DDCI) use in combination with levodopa as standard practice since the mid-1970s. Key to the treatment of PD symptoms is the maintenance of low and sustained levels of levodopa to reduce the severity and impact of response fluctuations. Through understanding the mechanism of action of catechol-O-methyltransferase (COMT) inhibitors, it is a logical step that adding a COMT inhibitor will optimise levodopa bioavailability in the periphery and increase the benefit of each levodopa dose. Opicapone is a COMT inhibitor indicated as adjunctive therapy to preparations of levodopa/DDCI in adult patients with PD and end-of-dose motor fluctuations that cannot be stabilised using this combination alone. Data from post hoc analyses of the pivotal BIPARK-I and -II studies reinforced the suitability of opicapone as a treatment option across the spectrum of motor fluctuations in PD. Real-world clinical data from the OPTIPARK study further supported the efficacy and safety of opicapone 50 mg, confirming its clinical utility as an adjunct to levodopa/DDCI in patients with PD and motor fluctuations.Introduction from The Chair
Professor Bastiaan R. Bloem
Presented here are highlights of a virtual satellite symposium from the European Academy of Neurology (EAN) 2020 Virtual Congress. The optimisation of levodopa bioavailability in the periphery in patients with PD was reviewed, with a focus on the role of COMT inhibition. The latest data on the efficacy and safety of opicapone 50 mg as an adjunct to levodopa/DDCI in the management of the full spectrum and duration of motor fluctuations were then discussed, together with emerging real-world experience of opicapone use.
Improving Levodopa Delivery: Let’s Move to The Periphery
Professor Werner Poewe
Levodopa is rightfully referred to as a revolutionary drug in the treatment of PD, and after five decades it is still considered the gold standard of symptomatic efficacy for the treatment of motor symptoms.1 Almost all patients with PD will eventually require levodopa and the first few years of treatment of newly diagnosed cases are often referred to as the ‘honeymoon period’, during which symptoms often improve to near normality.2 This period is the most rewarding for both patients and the treating neurologist, but unfortunately becomes compromised when patients develop motor response oscillations such as end-of-dose wearing-off and levodopa-induced dyskinesias.2-4
Risk Factors for Motor Complications Associated with Levodopa Therapy
Risk factors for the development of motor complications include a younger age at disease onset, levodopa dose, and disease duration.5-7 Findings from both the ELLDOPA and the STRIDE-PD studies showed a dose–response relationship for both the development of dyskinesias and wearing-off motor fluctuations.6,8 In STRIDE-PD, the frequency of dyskinesia and wearing-off after 208 weeks was greatest in patients treated with >600 mg/day of levodopa (55.8% and 72.6%, respectively).6
More recent findings provide clear evidence that motor fluctuations and the development of levodopa-induced dyskinesia are associated with a longer duration of PD and with increased levodopa dose rather than duration of exposure to levodopa therapy.7 Cilia et al.7 were able to compare the temporal evolution of motor complications in response to levodopa in two cohorts of patients with PD: one from Ghana, where access to PD medication is limited, and diagnosis and initiation of levodopa therapy are often delayed by many years relative to disease onset, and the other from Italy, where levodopa is initiated soon after diagnosis. Both cohorts were followed for 4 years and despite much later introduction of levodopa therapy relative to disease onset in the Ghanaian cohort
(4.2 years versus 2.4 years in Italy), wearing-off and dyskinesia occurred at a similar disease duration in both populations: wearing-off at 5.5–6.0 years and dyskinesia at 6.5–7.0 years,7 thus clearly revealing duration of disease as more relevant than duration of exposure to levodopa.
The mechanisms underlying the development of levodopa response fluctuations are multifactorial, but perhaps the most important factor contributing to the end-of-dose wearing-off phenomenon is levodopa’s short half-life, which is a result of its metabolism by aromatic L-amino acid decarboxylase and COMT in the periphery.9,10 Drugs capable of inhibiting levodopa decarboxylation in the periphery, thereby increasing its bioavailability in the brain, have led to the use of DDCI in combination with levodopa as standard practice in patients with PD.9
The Role of Catechol-O-Methyltransferase Inhibition in Improving the Bioavailability of Levodopa
To further prevent the peripheral metabolism of levodopa, thereby enhancing its availability in the periphery, transportation through the blood–brain barrier, and subsequent duration of therapeutic effect, COMT inhibition in combination with levodopa/DDCI was investigated as far back as the 1970s.9,11 Metabolism of levodopa by COMT is a second, important degradation pathway leading to the conversion of levodopa to 3-O-methyldopa; inhibiting COMT enables prolongation of levodopa half-life.9
The first-generation COMT inhibitors had little value as pharmacological agents because of their unfavourable pharmacokinetics, poor selectivity, or toxicity.11 Second-generation COMT inhibitors were introduced into clinical practice in the late 1990s. Tolcapone, with its central and peripheral mode of action, was the first-in-class COMT inhibitor to be approved for clinical use in 1997 as add-on therapy to levodopa. However, as a result of liver toxicity it was withdrawn from many European markets and its use is now second-line under strict liver function monitoring.9,12 Entacapone, a peripheral COMT inhibitor, was approved for clinical use in 1998 and has been used routinely as add-on therapy to levodopa/DDCI or in a triple combination tablet (levodopa/carbidopa/entacapone) in patients with motor fluctuations.9,13 Common adverse events (AE) reported with entacapone and tolcapone therapy related to increases in plasma concentration of levodopa include dyskinesia, nausea, and orthostatic hypotension. Furthermore, some patients experience diarrhoea and urine discolouration with entacapone therapy.12,13
The efficacy of enhancing peripheral availability of levodopa/DDCI with COMT inhibition to reduce wearing-off fluctuations has been demonstrated in multiple randomised controlled trials in patients with PD and motor complications.14
Second-Generation Catechol-O-Methyltransferase Inhibitors
Limitations of the efficacy of entacapone,15 as well as side effects such as troublesome diarrhoea,15 have stimulated research into new types of COMT inhibitors, which has recently led to the development of opicapone, a long-acting, purely peripheral COMT inhibitor.16 Opicapone has a high binding affinity for COMT, and a constant, slow dissociation rate of the enzyme–substrate complex, leading to a long (>24 hour) duration of action in vivo.16,17 A pharmacokinetic study found that after 11 days of dosing, opicapone 50 mg decreased 3-O-methyldopa exposure to a greater degree than entacapone 200 mg: mean Cmax was 361 ng/mL and 785 ng/mL, respectively.18 Improving oral levodopa delivery through COMT inhibition is now an established adjunct to levodopa/DCCI to manage wearing-off. How opicapone has advanced COMT inhibitor therapy was discussed by Prof Ebersbach in the second presentation of the symposium.
Opicapone’s Latest Insights
Professor Georg Ebersbach
Opicapone is a COMT inhibitor indicated as adjunctive therapy to preparations of levodopa/DDCI in adult patients with PD and end-of-dose motor fluctuations who cannot be stabilised on those combinations.17
The clinical efficacy and safety of opicapone as an adjunct therapy to levodopa has been demonstrated in two large, Phase III, multinational, randomised, double-blind studies with open-label extension periods. BIPARK-I was an active comparator (entacapone) and placebo-controlled study (N=600), and BIPARK-II a placebo-controlled study (N=427).19-21 In BIPARK-I, the primary endpoint was change in baseline to end of study treatment in absolute OFF time. Treatment with opicapone 50 mg was superior to placebo (mean difference in change from baseline: -60.8 min; 95% confidence interval [CI]: -97.2 to -24.4; p=0.0015) and noninferior to entacapone (mean difference: -26.2 min; 95% CI: -63.8 to 11.4; p=0.0051 for noninferiority test).19 In BIPARK-II, the primary efficacy outcome in the double-blind phase was change from baseline in absolute OFF time versus placebo. The adjusted treatment difference versus placebo was significant for opicapone 50 mg (treatment effect: -54.3 min; 95% CI: -96.2 to -12.4; p=0.008).21 Opicapone was generally well tolerated with the most common AE associated with opicapone treatment including dyskinesia, insomnia, constipation, and dry mouth.19,21
Opicapone in Patients Who Have Recently Developed Motor Fluctuations
Building on these Phase III data, exploratory post hoc analyses evaluated the efficacy and safety of opicapone in patients with PD treated with levodopa/DDCI with ≤1 year duration of motor fluctuations (recent motor fluctuations [RMF]), as well as >1 year duration of motor fluctuations (long-standing motor fluctuations [LMF]).22
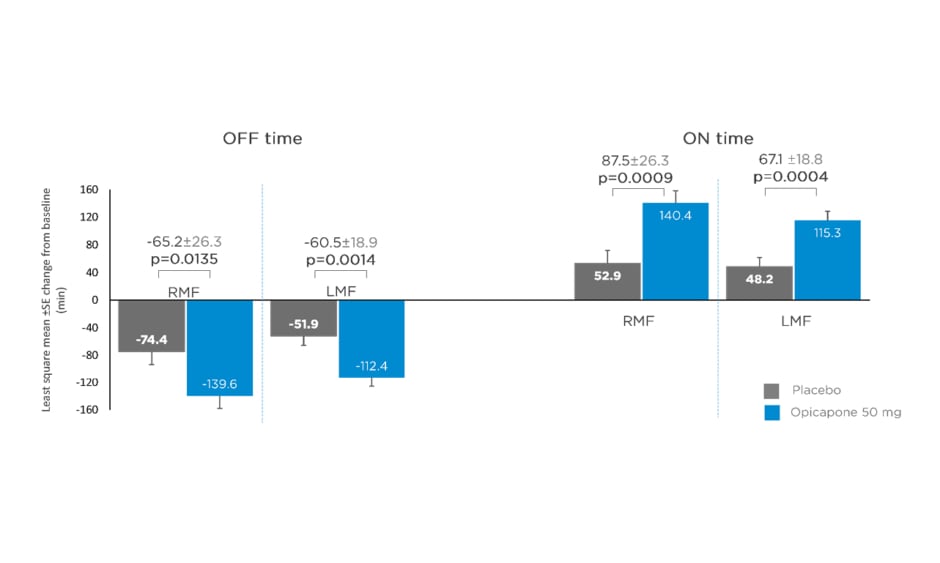
Data from matching treatment arms in BIPARK-I and -II were combined for the placebo and opicapone 50 mg groups and analysed. Key baseline patient characteristics, including age, disease duration, and daily OFF time, were similar for opicapone (RMF: n=85; LMF: n=162) and placebo (RMF: n=71; LMF: n=174) groups in both RMF and LMF patients.22 Mean daily levodopa dose was slightly higher for LMF (placebo: 742.3 mg; opicapone 50 mg: 739.3 mg) compared with RMF (placebo: 585.4 mg; opicapone 50 mg: 616.6 mg).22 Changes in absolute OFF and ON time were significantly greater for opicapone versus placebo in both RMF and LMF. Opicapone reduced absolute OFF time by approximately 1 hour for RMF and LMF versus placebo (least squares mean RMF: -65.2 min; least squares mean LMF: -60.5 min) (Figure 1).22
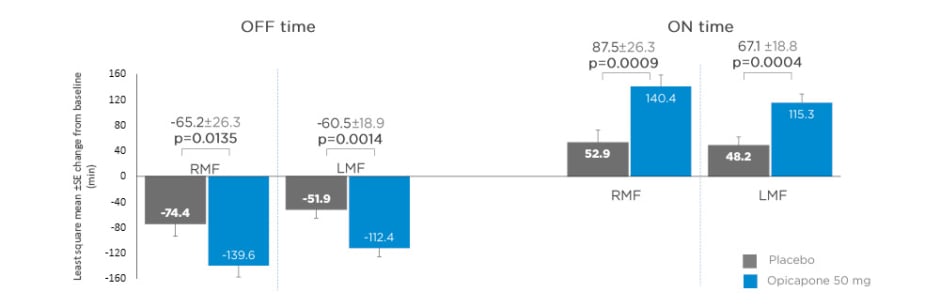
Figure 1: Changes from baseline in absolute OFF and ON time in recent and long-standing motor fluctuations on opicapone 50 mg or placebo.
LMF: long-standing motor fluctuations; RMF: recent motor fluctuations; SE: standard error.
Adapted from Ebersbach G et al.22
Dyskinesia was the most frequently reported potentially related treatment-emergent AE (TEAE), with a 2-fold increase in dyskinesia for late versus recent motor fluctuators in the opicapone groups (23.5% versus 11.8%).22 This could be because of longer disease duration and higher daily levodopa dose in the late fluctuators.22 These data support the use of opicapone regardless of duration of onset of motor fluctuations, but with a lower incidence of dyskinesia in the first year of motor fluctuations.22
Opicapone As First Add-On to Levodopa/Dopa-Decarboxylase Inhibitor in Patients With Motor Fluctuations
A post hoc analysis evaluating opicapone as first add-on in patients with PD with end-of-dose motor fluctuations treated with levodopa/DDCI only at baseline (i.e., without dopamine agonists or monoamine oxidase-B inhibitors) was conducted in 127 patients.23 Baseline characteristics in the opicapone (n=68) and placebo (n=59) groups were comparable, with mean levodopa dose 730.3 mg and 718.3 mg/day, respectively.23 Opicapone significantly improved OFF and ON time in patients treated with levodopa/DDCI alone compared with placebo, with mean changes from baseline in absolute OFF time reduced by 68.8 min (p=0.0161), and ON time increased by 79.8 min (p=0.0049) (Figure 2).23
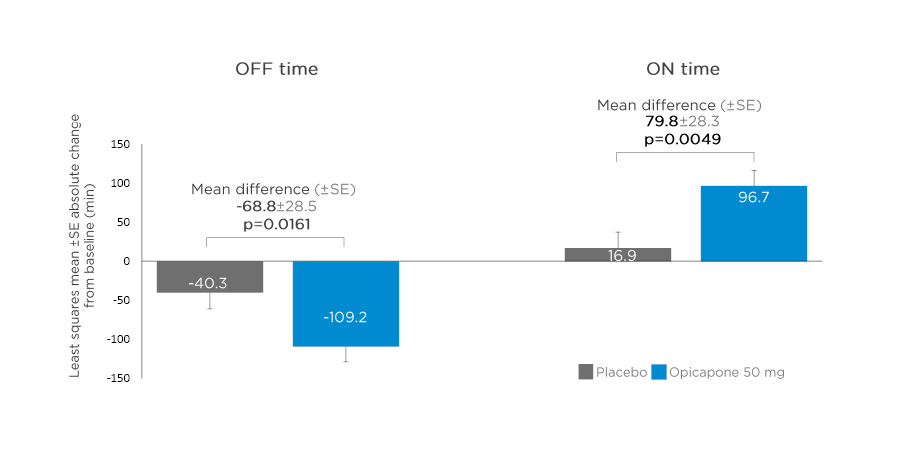
Figure 2: Changes from baseline in absolute OFF and ON time in patients treated with levodopa/dopa-decarboxylase inhibitor alone* and opicapone 50 mg as first-line adjunctive therapy.
*Without dopamine agonists or monoamine oxidase-B inhibitors.
SE: standard error.
Adapted from Ferreira JJ et al.23
The incidence of potentially related TEAE leading to discontinuation were comparable for opicapone 50 mg (n=5 [7.4%]) and placebo (n=5 [8.5%]).23 The most frequently reported (≥5% of patients) potentially related TEAE were dyskinesia (opicapone: n=8 [11.8%]; placebo: n=1 [1.7%]), constipation (opicapone: n=4 [5.9%]; placebo: n=0 [0.0%]), and nausea (opicapone: n=1 [1.5%]; placebo: n=4 [6.8%]).23 These data show that opicapone is effective and generally well tolerated as a first-line adjunctive therapy in levodopa-treated patients with PD and motor fluctuations.23
Opicapone in Patients With Advanced Complications of Levodopa Treatment
A further post hoc analysis was conducted to investigate levodopa dose reductions seen with opicapone 50 mg in the BIPARK-I and -II studies.24 Opicapone efficacy was assessed in levodopa-treated patients with PD whose levodopa dose was reduced during the double-blind adjustment period. Overall, 41 out of 265 patients treated with opicapone 50 mg had levodopa dose reductions, either as a proactive dose reduction (n=11), or because of dopaminergic AE (n=30).24
Patients with these levodopa dose reductions had a longer mean (standard deviation [SD]) disease duration (10.1 [4.6] years) than the overall opicapone 50 mg population (7.7 [4.3] years; n=265), and higher mean (SD) daily doses of levodopa: 842 (344) mg/day and 698 (322) mg/day, respectively.24 Although the mean daily levodopa dose decreased by an average of 23.4% (842 mg to 650 mg), these patients still experienced motor response and quality of life (QoL) improvements from baseline in absolute OFF time (mean decrease of 131.2 min), ON time (increase of 125.4 min), Unified PD Rating Scale (UPDRS) II and III scores (-3.3 and -1.7, respectively), and PD Questionnaire (PDQ) 39 items (PDQ-39 score; -2.8).24 These findings show that dopaminergic AE emerging with opicapone can be managed by reduction of levodopa dose and that improvements for patients are still provided after reduction of levodopa.24
Opicapone as a Treatment Option Across the Spectrum of Motor Fluctuations
In summary, data from the BIPARK-I and -II post hoc analyses have shown that opicapone 50 mg improves OFF and ON time in patients with RMF (≤1 year of motor fluctuations) as well as patients with LMF, and that there is a lower incidence of dyskinesia in RMF compared with LMF.22 Opicapone significantly improved OFF and ON time as a first adjunctive therapy in patients with motor fluctuations.23 Dopaminergic AE emerging with opicapone can be managed by levodopa dose reduction, with improvements in motor response and QoL still present.24 Opicapone was generally well tolerated in all subgroups.22-24
Opicapone in Clinical Practice: OPTIPARK, a Phase IV Open-Label Study
Real-world evidence from the recently published OPTIPARK study added further credence to the utility of opicapone 50 mg in clinical practice.25 Full details of the OPTIPARK study have been described elsewhere.25 In brief, OPTIPARK was a Phase IV, real-world, prospective, open-label, uncontrolled, single-group trial in adults with PD with wearing-off motor fluctuations conducted in the UK (6 months) and Germany (3 months). Main inclusion criteria were Stage I–IV of disease severity (modified Hoehn and Yahr Scale) in the ON state, and treated with 3–7 daily doses of levodopa/DDCI or levodopa/DDCI/entacapone. Total daily levodopa/DDCI dose could be adjusted according to the individual’s condition throughout the trial (except on Day 1). Patients treated with entacapone before trial entry were to discontinue entacapone at the baseline visit; patients previously or currently treated with tolcapone and/or opicapone were excluded from the study.25
The primary efficacy endpoint was Clinician’s Global Impression of Change (CGI-C) at 3 months, and secondary endpoints included Patient’s Global Impression of Change (PGI-C), Wearing-off Questionnaire (WOQ)-9 assessments, UPDRS, PDQ-8, and Non-Motor Symptoms Scale (NMSS).25
Patient characteristics
A total of 495 patients were included in the safety set. Mean (SD) age was 67.7 (8.98) years, disease duration was 8.5 (4.97) years, and duration of motor fluctuations was 2.5 (3.16) years. Mean (SD) total levodopa daily dose was 580.1 (289.1) mg. Overall, 393 patients completed 3 months; 109 patients terminated the study prematurely, mainly due to nonserious AE (n=76).25
Efficacy results
After 3 months’ treatment with opicapone 50 mg in a clinical setting, there were improvements in global PD condition: 71.3% of patients showed clinical improvement as rated by the CGI-C (primary endpoint), with 43% reported as much or very much improved,25 and 76.9% self-reported a clinical improvement rated by PGI-C (secondary endpoint), with 48.1% of patients reporting they were much or very much improved (Figure 3).25,26
Opicapone also significantly improved UPDRS Part II (activities of daily living) and III (motor) scores, QoL (PDQ-8 total score), and Non-Motor Symptoms Scale (NMSS) scores after 3 months (Figure 4).25,26
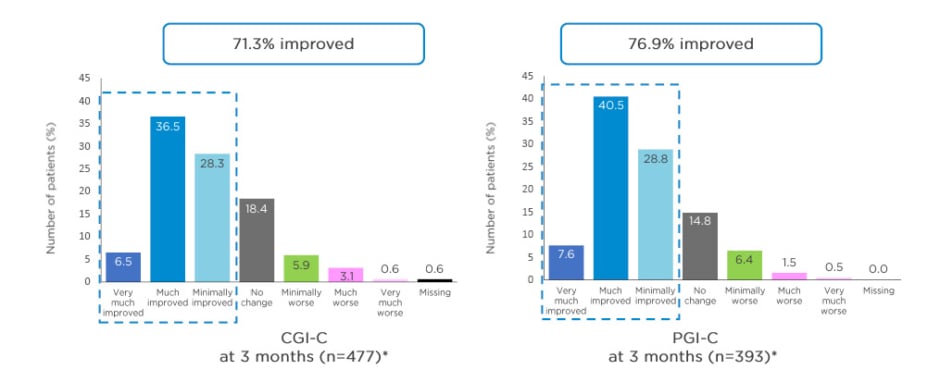
Figure 3: Clinician’s Global Impression of Change (CGI-C) and Patient’s Global Impression of Change (PGI-C) at 3 months in OPTIPARK.
*Data from full analysis set. Missing values for CGI-C at visit 4 were imputed using the last observation carried forward method.
Adapted from Reichmann et al.; OPTIPARK investigators.25,26
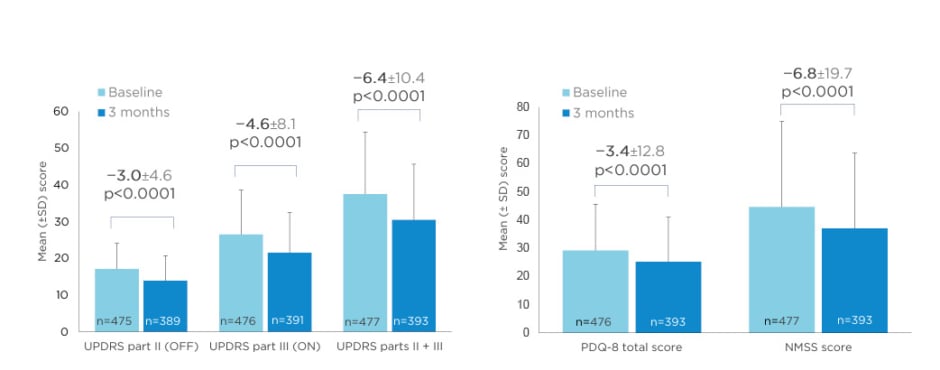
Figure 4: Activities of daily living and motor scores (UPDRS Parts II and III), and Quality of Life (PDQ-8) and Non-Motor Symptoms Scale (NMSS) scores at 3 months in OPTIPARK.
NMSS: Non-Motor Symptoms Scale; PDQ: Parkinson’s Disease Questionnaire; SD: standard deviation; UPDRS: Unified Parkinson’s Disease Rating Scale.
Adapted from Reichmann et al.; OPTIPARK investigators.25,26
Safety results
The majority of drug-related TEAE were reported during the first week.27 In the 74.9% of patients who experienced TEAE, the majority were mild or moderate in severity. Dyskinesia was the most common at least possibly-related TEAE (11.5%) but had a low impact on patient discontinuation (1.0%). The most common reason for withdrawal was nausea, affecting 2.0% of patients.25 Overall, observed AE in this large open-label study were comparable to AE data from the two pivotal clinical trials.19,21
Conclusion
In summary, these real-world clinical data support the efficacy and safety of opicapone 50 mg observed in Phase III studies and post hoc analyses. OPTIPARK confirms the clinical utility of opicapone 50 mg as an effective and generally well-tolerated adjunct option in patients with PD with motor fluctuations.25
Symposium Panel Discussion
Professor Bas Bloem, Professor Werner Poewe, and Professor Georg Ebersbach
At the end of the well-attended live-streamed satellite symposium, delegates were able to ask questions to the panel. A selection of questions pertinent to the clinical use of opicapone 50 mg as an adjunct to levodopa/DDCI in the management of the full spectrum and duration of motor fluctuations in patients with PD, which were answered by the expert speaker panel, are presented here.
Opicapone Therapy and Onset of Efficacy in Improving Motor Fluctuations
In response to the question: “How long is the wait before adjunct opicapone therapy starts to take effect, and in order to see an improvement in motor fluctuations?,” Prof Ebersbach replied that the first effects can be expected to appear within the first few days of treatment, with the full effect becoming present and obvious after 1–2 more weeks of opicapone treatment.28
In a follow-up question enquiring when to reduce the dose of levodopa following the addition of opicapone (i.e., should this be an immediate reduction of levodopa, or is it best to wait for dyskinesia to happen before reducing the levodopa dose?), Prof Ebersbach responded that this depends on the risk profile of the individual patient. If a patient is prone to dyskinesia with any rise in levodopa dose, then the dose could be lowered prophylactically (i.e., at the same time as introducing the opicapone). However, in a patient who has not previously experienced dyskinesia, the levodopa dose does not necessarily need to be lowered in anticipation of this possible side effect, which only occurs in a subgroup of patients following the addition of opicapone (20.4% with opicapone 50 mg in the pooled double-blind Phase III trials [n=265]29). According to the clinical condition of the patient, it is often necessary to adjust the daily dose of levodopa within the first few days to first weeks after initiating treatment with opicapone.17 In 41 out of 265 patients treated with opicapone 50 mg who had levodopa dose reductions during the double-blind phase of BIPARK-I and -II, the mean daily levodopa dose decreased by an average of 23.4% (from 842 mg to 650 mg).24
Conversion Factor for Levodopa and Opicapone
With levodopa as the gold standard, conversion factors can be used to calculate levodopa-equivalent doses (LED) for comparison of drug regimens. Prof Poewe’s response to a question about the LED conversion factor for opicapone was that it is proposed to be 1.5, in comparison with entacapone’s LED conversion factor of 1.3. Thus, opicapone’s LED is 140–150 mg for a 100 mg levodopa dose.30
Switching From Entacapone to Opicapone
Prof Poewe’s answer to a question asking for a recommendation on how to switch from entacapone to opicapone came from experience in the BIPARK-I study (Figure 5).31
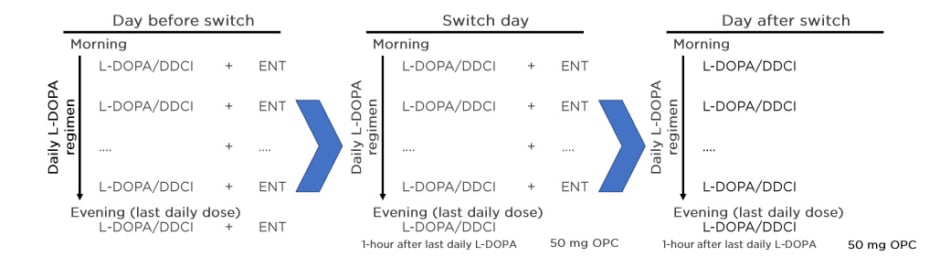
Figure 5: Practical switch from entacapone to opicapone 50 mg.
DDCI: dopa-decarboxylase inhibitor; ENT: entacapone; L-DOPA: levodopa; OPC: opicapone.
Adapted from Ferreira J et al.31
Entacapone’s half-life is similar to the half-life of levodopa, so the switch can be made in 1 day. On the day of the switch, the last daily dose of levodopa/DDCI should be taken without entacapone, and opicapone 50 mg started on that same night, at least 1 hour apart from the last dose of levodopa/DDCI.31 This recommendation was confirmed by Prof Ebersbach.
Long-Term Effectiveness of Opicapone
In reply to a question asking whether the effect of opicapone decreases after several months of treatment as a result of loss of intrinsic efficacy, or whether this is attributable to worsening of the underlying PD, Prof Poewe responded by highlighting evidence from the pooled analysis of data from BIPARK-I and -II, and their associated open-label extension studies. Opicapone does not give any indication of loss of benefit over time and persistent benefit has been shown in the open-label extension after 1 year.32 In Prof Poewe’s experience, there are many reasons for reduced benefit with treatments in general, including disease progression, psychology, and low compliance.
Opicapone as Adjunct Therapy to Levodopa in Patients With Parkinson’s Disease
How would you describe the ideal patient for opicapone? In response to this question, Prof Ebersbach outlined two types of suitable patients. In a patient with recent motor fluctuations, the addition of opicapone is usually straightforward and often does not need any adjustment to co-medication.
On the other end of the spectrum, patients with severe motor response fluctuations are likely to benefit but, in this situation, levodopa dose may have to be adjusted.22-24
CLICK BELOW TO VIEW THE FOLLOWING
Opicapone▼ Prescribing Information