Meeting Summary
Chronic kidney disease (CKD)-associated pruritus (CKD-aP) is diagnosed after exclusion of other potentially itch-causing conditions and factors. CKD-aP can be a life-disrupting condition for many who experience it, with itch affecting quality of life (QoL) factors and leading to sleep disruption, lower self-confidence, and depression in some. Despite this, CKD-aP is under-recognised, with reports coming predominantly from patients themselves, as opposed to all patients with CKD undergoing haemodialysis being systematically assessed for CKD-aP using validated screening tools. Treatment options for CKD-aP are limited, with targeting of the possible causes or exacerbating factors of CKD-aP being the current choices. These include haemodialysis optimisation, moisturisers for dry skin, gabapentinoids for neuropathic aetiology, and opioid receptor antagonists/agonists for potential opioid imbalance. The k-opioid receptor agonist difelikefalin is one of the first medications trialled to specifically address CKD-aP. Two large studies of this agent, KALM-1 and KALM-2, showed its efficacy in reducing itch intensity and improving itch-related QoL factors for haemodialysis patients with CKD-aP, as well as a tolerable safety profile. Here, the speakers discuss four abstracts related to patients with CKD-aP undergoing haemodialysis, presented at recent congresses. The first, presented by James Burton, surveyed nephrologists regarding CKD-aP incidence, assessment, diagnosis, and treatment. Two studies assessed pooled data from the difelikefalin KALM-1 and KALM-2 clinical trials regarding safety and efficacy of this agent both in the initial 12-week placebo-controlled trial period, as presented by Joel Topf, and in a 52-week open-label extension study, as presented by Steven Fishbane. Finally, Daniel Weiner discussed results of a 12-week open-label study, providing insights into potential real-world effectiveness of difelikefalin. These studies collectively confirmed the utility of difelikefalin in reducing itch intensity and improving itch-related QoL while showing favourable tolerability.
Introduction
CKD-aP necessitates a differential diagnosis to ascertain that the itching a patient is experiencing is related to renal insufficiency and other potential pruritus-causing factors; conditions are examined, treated if required, and ruled out.1 The causative factor of CKD-aP is not fully known but is thought to be related to opioid imbalance; systemic inflammation; imbalance of kidney-disease-associated factors, such as parathyroid hormone, calcium, and phosphorus; and neurogenic pruritus, potentially leading to peripheral neuropathy.1-3
The itch of CKD-aP may occur symmetrically on the body but it can also be localised to a single place, with the most frequent being on the back, face, and shunt arm.4 Although CKD-aP can be exacerbated by dry skin, and while complications of itching may include focal papules, skin crusting, and erosions, none of these factors are diagnostic of the condition.1 Diagnostic difficulties may also arise because severity, location, and dermatological presentation may not only vary by patient, but within a patient over time, and may not necessarily be associated with times before, during, or after haemodialysis.5 CKD-aP can also be exacerbated by factors such as temperature extremes, physical exercise, and showering.1
The Dialysis Outcomes and Practice Patterns Study (DOPPS), an international prospective cohort survey of 23,264 patients with CKD being treated at haemodialysis centres, examined several factors associated with QoL. The study found that two-thirds of those surveyed experienced CKD-aP, and over one-third (37%) were bothered at least moderately.6 However, despite the high prevalence of CKD-aP, it is an under-recognised condition.5,6 This is important to note as occurrence of CKD-aP is associated with reduced health-related QoL, poor sleep, and depression.4-7
Here, the speakers discuss four presentations of studies from leading nephrology teams. The first, an oral presentation regarding CKD-aP recognition and unmet needs, was delivered at the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) 58th Congress (virtual), 5−8 June, 2021, by Burton.8 Next the speakers discuss two posters, presented at the National Kidney Foundation Spring Clinical Meeting, 6−10 April, 2021, by Topf9 and Fishbane,10 which detailed post-hoc analyses of the clinical trials of difelikefalin, a novel, selective, peripheral k-opioid receptor (KOR) agonist in development for CKD-aP. Finally, an oral presentation delivered at the 2021 ERA-EDTA Congress by Weiner11 regarding a study that examined difelikefalin efficacy and safety in a Phase III, open-label, multicentre design is discussed.
Current Challenges and Unmet Needs Relating to Chronic-Kidney-Disease-Associated Pruritus Diagnosis
Weiner highlighted in his talk how CKD-aP is often subject to under-reporting. “It’s remarkably common,” he said, “once you start asking about it, you’re seeing this in probably a third or so of patients where it’s significant and can be disruptive.”11 This was confirmed in the survey presented by Burton where nephrologists estimated that 34% of their haemodialysis patients had CKD-aP, over half of which (55%) had moderate-to-severe symptoms. This real-world survey, carried out between May and July 2020, included 301 nephrologists treating at least five haemodialysis patients with CKD-aP from Australia (n=30), France (n=50), Germany (n=56), Italy (n=58), Spain (n=55), and the UK (n=52). Participants responded to questions using a 7-point scale from 1 (do not agree at all) to 7 (strongly agree).8
Of note, while DOPPS data showed similar levels of self-reporting patients were moderately to extremely bothered by ‘itchy skin’ (37%), another 30% reported that they were ‘somewhat’ bothered, meaning the overall prevalence of CKD-aP may be higher than nephrologists realise.6 This is reflected in the survey revealing that 75% of responding nephrologists agreed that CKD-aP is underdiagnosed in haemodialysis patients, and most (80%) agreed that diagnosis is usually patient driven, such as when the patient mentions itching as an issue.8
Diagnosis of Chronic-Kidney-Disease-Associated Pruritus
The above findings reveal an unmet need for proper screening and diagnosis of CKD-aP. Indeed, the survey suggested that one of the main barriers to screening was lack of diagnostic guidelines (74% of respondents agreed). Despite reasonably high levels of incidence and severity of CKD-aP in their patients, most (79%) of nephrologists participating in the survey reported that they do not use any formal itch scales in clinical practice, with only 42% agreeing that their centre had a systematic approach to CKD-aP screening. This may have been because only 47% thought that CKD-aP is easy to diagnose using clinical observation alone.8
In the survey, 71% of nephrologists agreed that standardised scales were needed for CKD-aP diagnosis.8 There are a number of tools that can be used to assess itch severity, such as CKD-aP-directed visual analogue and verbal rating scales and the Kidney Disease Quality of Life-Short Form.1 In clinical trials, a variety of scales have been used that may also be of use in clinical practice, including the Worst Itching Intensity Numerical Rating Scale (WI-NRS), a validated 11-point scale ranging from 0 (no itching) to 10 (worse itching imaginable). On this scale, mild itching is defined as a score 0−3, moderate is 4−6, and severe is 7−10.4,12 Another example is the Skindex-10 Scale, which was specifically developed to assess pruritus-related QoL in haemodialysis patients with CKD-aP across three domains (disease, mood/emotional distress, social functioning). Total scores have a range of 0−60, with higher scores indicating worse itch-related QoL.4 Finally, the 5-D Itch Scale assesses five dimensions of itch during a 2-week recall period: duration, degree, direction, disability, and distribution. Scores range 5−25, with higher scores indicating worse itch-related QoL.13
Current Treatment Options for Chronic-Kidney-Disease-Associated Pruritus
According to Burton, along with the need for new guidelines, “There is an urgent need to develop new targeted treatment options that are both effective and well tolerated.” The lack of such guidelines and therapies, he stated, “leads to an inconsistent, fragmented approach to management.” Indeed, in the survey, 43% of respondents declared they had no specific therapy of choice and nearly three-quarters (72%) agreed that treatment options for CKD-aP are limited. Many felt major treatment improvements were needed in terms of improved efficacy for reduction of itch intensity (62%) and ability to improve a patient’s QoL (57%).8
Current CKD-aP treatments include tackling the itch and potential causes of itch exacerbation. While CKD-aP occurrence is not necessarily concordant with haemodialysis, it is recommended to assess the quality of every part of this process.14 This action is reflected in the survey, where 33% of the nephrologists cited ‘haemodialysis optimisation’ as their therapy of choice.8 As dry skin occurs in many haemodialysis patients15 and can contribute to CKD-aP severity, treatment of such is recommended.1 The survey, however, revealed that only 5% of participating nephrologists put moisturisers and emollients as their therapy of choice.8 Gabapentinoids, used by 5% of the surveyed nephrologists,8 may decrease itching for some patients, but use must be weighed against potential side effects such as dizziness and drowsiness.1
Other treatments, which 7% of survey respondents cited using without naming them,8 include immunomodulators, such as ultraviolet-B therapy, γ-linolenic acid and mast-cell stabilisers, and treatments targeting opioid imbalances, such as KOR agonists and µ-opioid receptor antagonists.1 While it may be thought that antihistamines could be of use, with 9% of survey respondents listing these as their therapy of choice,8 they are not shown to be successful for CKD-aP.1
In the survey, only one option could be named ‘therapy of choice’; however, another analysis presented by Burton, which captured patient report-form data from 1,435 CKD-aP haemodialysis patients from Australia and Europe, found that approximately 85−90% of patients on at least their second line of CKD-aP therapy were prescribed combinations of different treatments. These findings, according to Burton, “highlight the uncertainties that physicians face relating to best treatment practice.”8
Difelikefalin As a Treatment for Chronic-Kidney-Disease-Associated Pruritus: Clinical Studies and Pooled Analysis
One such treatment that may tackle CKD-aP is the KOR agonist difelikefalin, the anti-pruritic effect of which is thought to occur via activation of KORs located on peripheral sensory neurones and immune cells.16,17 Difelikefalin doesn’t bind to µ-opioid receptors or any other known receptors16 and has the advantage of limited central nervous system penetration,16 so, according to Weiner, “it should not have some of the side effects that we see with other opioid receptor agonists.”
The KALM-1 and KALM-2 studies were large, global Phase III trials in haemodialysis patients with CKD-aP.18,19 Both trials included adults (≥18 years for KALM-1, 18−85 years for KALM-2) undergoing haemodialysis three times per week for at least 3 months. In the 7-day run-in period prior to difelikefalin administration, patients needed a mean WI-NRS score of >4 (KALM-1) or ≥5 (KALM-2) to qualify for study inclusion. Randomisation was on a 1:1 basis, with patients receiving either 0.5 mg/kg difelikefalin or a placebo. Administration was via intravenous injection three times per week for 12 weeks. In KALM-1, 189 patients were randomised to difelikefalin and 189 to placebo; respective numbers in KALM-2 were n=237 and n=236.18,19
Results of the studies demonstrated significant improvements in itch intensity versus placebo, and the primary endpoint, percentage of patients achieving ≥3-point improvement in WI-NRS scores at Week 12, was met in both trials. Topf9 and Fishbane10 reported on analysis of pooled KALM-1 and KALM-2 data (difelikefalin: n=426; placebo: n=425). Baseline characteristics were similar between difelikefalin and placebo groups, with mean ages (standard deviation, SD) of 59.1 (12.4) and 58.3 (13.5) years and 58.5% and 60.7% being male, respectively. As KALM-1 only included patients from the USA, with KALM-2 extending to other countries, most patients in the pooled data were from the USA (difelikefalin group: n=335; placebo group: n=322), with others in KALM-2 from Eastern Europe (n=54, n=60, respectively, for difelikefalin and placebo groups), Western Europe (n=29, n=31, respectively) or Asia (n=8, n=12, respectively).
Patients, on average, had undergone haemodialysis for a mean (SD) of 4.6 (4.3) years for the difelikefalin group, and 4.9 (4.3) years for the placebo group. They reported experiencing CKD-aP for an average of 3.2 (4.0) and 3.3 (3.3) years, respectively, and 37.3% in the difelikefalin group and 38.4% in the placebo groups reported using an anti-itch medication prior to study initiation.9,10
For difelikefalin and placebo groups, respectively, baseline WI-NRS score means (SD) were almost identical: 7.2 (1.4) and 7.2 (1.5); as were those of the Skindex-10 total score means: 35.8 (14.7) and 36.0 (15.1); and 5-D itch score means: 16.8 (3.5) and 16.9 (3.5). It was also reported that 15.7% of the difelikefalin group and 15.3% of the placebo group experienced at least one specified medical condition prior to study start (including fall/fall-related fracture history, a confusional state, mental status change/alteration, disorientation, gait disturbance, or movement disorder).9,10
Efficacy of Difelikefalin
The post-hoc analysis carried out by Topf and colleagues had the achievement outcome of a ≥3-point improvement in weekly WI-NRS score, evaluated in subgroups, based on pooled baseline characteristics.9
Confirming the findings of the individual KALM studies, in the pooled population the percentage of patients who achieved a ≥3-point WI-NRS improvement rose throughout the study in both groups but was significantly greater with difelikefalin compared to the placebo at all timepoints from Week 1 (p<0.05) to Week 12 (p<0.001 for all other weeks). Additionally, significantly greater proportions of patients in the difelikefalin group compared to placebo achieved a complete response (≥80% of weekly WI-NRS scores equal to 0 or 1) from Week 3 to Week 12 (p<0.001 for Weeks 5, 7, and 11; p<0.05 for all other weeks). By Week 12, complete response was seen in approximately 12% of those in the difelikefalin group and 6% in the placebo group.9
Subgroup analysis (Figure 1) investigated whether results were influence by several study participant factors. Analysis showed that the odds ratio of a ≥3-point WI-NRS improvement being significantly in favour of difelikefalin compared to the placebo occurred regardless of sex, geographic location, or randomisation strata. Odds ratio differed in terms of those identified as ‘other’ race; though of note, the numbers for this group were low (n=85) compared with the other categories.9
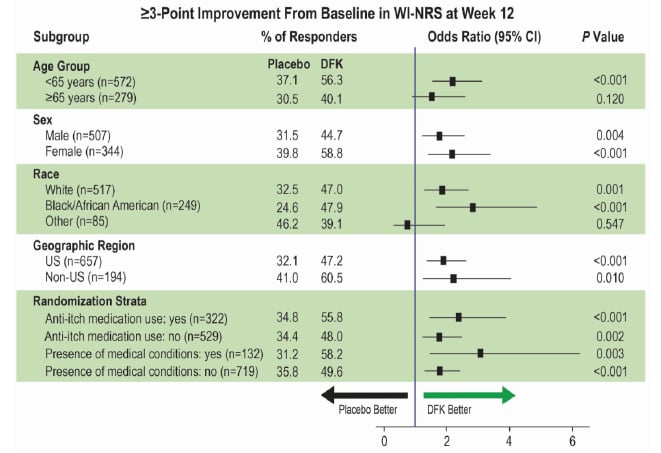
Figure 1: Subgroup analyses for WI-NRS response at Week 12 in the pooled KALM-1 and KALM-2 studies.⁹
Estimated percentages, odds ratios, and CIs were based on logistic regression with terms for treatment group, baseline WI-NRS score, region/study, use of anti-itch medication during the week prior to randomisation, and the presence of specific medical conditions. For analysis by geographic region and randomisation strata, corresponding subgroup variables were removed from the model, and study ID was included in the USA model. Missing values were imputed using multiple imputation under missing-at-random missing data assumption for interim patients and post-interim patients separately.
CI: confidence interval; DFK: difelikefalin; WI-NRS: Worst Itching Intensity Numerical Rating Scale.
This pooled analysis also analysed Skindex-10 scores. Here, while improvements were seen in both groups, a significantly greater achievement of clinically meaningful response (≥15-point improvement in total score) was observed with difelikefalin than with placebo at Weeks 4, 10 (both p<0.05), and 12 (p<0.001). This was additionally reflected in 5-D itch responses where a ≥5-point improvement in 5-D itch total score was shown in a significantly greater percentage of patients receiving difelikefalin than receiving placebo at all timepoints recorded (p<0.05 at Weeks 4 and 12; p<0.001 at Weeks 8 and 10).9
In conclusion, the authors noted that “These pooled efficacy findings suggest intravenous difelikefalin may play an important role in the care of CKD patients undergoing haemodialysis by reducing itch and improving QoL.”9
Long-Term Efficacy of Difelikefalin
While the Topf poster highlighted findings of the first 12 weeks of the KALM-1 and KALM-2 pooled studies, Fishbane and colleagues investigated the pooled data over both this trial period and a ≥52-week open-label extension (OLE) period. During the OLE, patients continued to receive 0.5 mg/kg difelikefalin three times per week or started to if they had previously received the placebo.
Of the total 796 patients in the pooled population in this analysis, 84 from the placebo-controlled period were not included in the OLE because they were not eligible or chose not to enter this phase. In KALM-2, in addition to the patients who discontinued from the OLE, 313/399 patients could not complete the 52-week OLE due to the sponsor’s decision to stop the study for reasons unrelated to safety or lack of drug effect.10
As can be seen from Figure 2, the improvement shown with difelikefalin during the double-blind period was maintained throughout all 52 weeks of the OLE. For those initially receiving the placebo but switched to difelikefalin, scores fell by the highest margin in the first 4 weeks of administration then more slowly, but with a continued decrease over the rest of the OLE period where the clinically meaningful difference of at least a 5-point change from baseline was retained and widened by Week 52.10
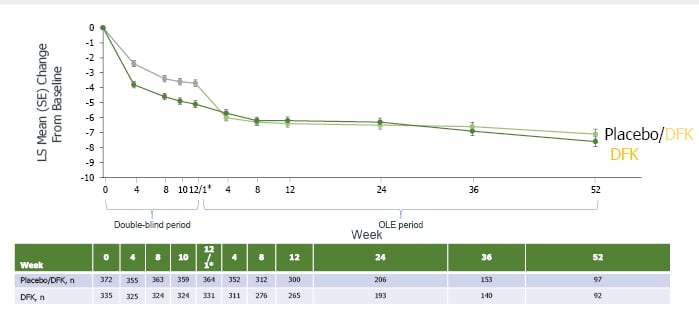
Figure 2: Mean improvement in 5-D Itch total score.10
*Week 12 of double-blind period; Week 1 of OLE period. The 2-week discontinuation period in KALM-1 is not pictured. DFK: difelikefalin; LS: least squares; OLE: open-label extension; SE: standard error.
The authors concluded that for haemodialysis patients with CKD-aP, for itch-related symptoms and QoL, difelikefalin demonstrated maintenance of efficacy over 1 year of use.
Safety of Difelikefalin
Fishbane and colleagues also examined safety data over the initial, placebo-controlled, and OLE study periods. This was evaluated based on adverse events, physical examinations, vital signs, ECGs, and clinical laboratory tests. Treatment-emergent adverse events (TEAE) were patient-reported in the OLE period. The safety population was defined as randomised patients who received at least one dose of their study drug during the 12-week placebo-controlled period and/or during the OLE period.10
Data were presented as incidence rate/1,000 patient-years (IR/1,000 pt-yrs) calculated as 1,000 times the number of events divided by total patient-years of exposure. During the 12-week placebo-controlled period, it was calculated that difelikefalin (n=424) was administered for a total of 98.0 pt-yrs and the placebo (n=424) for 101.1 pt-yrs. During Weeks 0−64 (12-week placebo-controlled period + OLE period), difelikefalin administration (n=796) was calculated to be for 537.4 pt-yrs.10
For difelikefalin, 57.5% of patients during the placebo-controlled period and 53.6% in the OLE period reported a TEAE, compared with 52.6% for the placebo. Incidence rates of TEAEs in the placebo-controlled and OLE periods were similar, with most judged to be mild–moderate. The IR/1,000 pt-yrs for at least one TEAE were similar in the difelikefalin group (10,862.9) compared with the placebo group (9,597.8); for the placebo-controlled + OLE period, this was lower for difelikefalin, with a figure of 8249.6. IR/1,000 pt-yrs calculated for experiencing at least one non-fatal serious TEAE were also similar for the 12-week difelikefalin (2,040.0) and placebo (1,860.2) groups, with the Weeks 0−64 calculation for difelikefalin being even closer to the placebo at 1,829.3. For experiencing a TEAE leading to discontinuation, IR/1,000 pt-yrs were 428.4, 395.8, and 174.9, respectively.10
The U.S. Renal Data System (USRDS) reports an incident rate of adverse events leading to death in haemodialysis patients of 164.6/1,000 pt-yrs.20
For the pooled data, IR/1,000 pt-yrs for adverse events leading to death were much lower in the initial 12-week trial period, with figures of 30.6 for the difelikefalin group, 49.5 for the placebo group, with IR/1,000 pt-yrs of 68.9 in the difelikefalin placebo-controlled + OLE period.10
Also highlighted in this poster were the most commonly reported TEAEs (≥2% during the placebo-controlled period and ≥1% higher incidence with difelikefalin versus placebo) in the pooled analysis, where IR/1,000 pt-yrs for the most common TEAEs with difelikefalin 12 weeks/placebo-controlled + OLE periods or placebo were, respectively: diarrhoea (469.2/273.6, 267.2); dizziness (316.2/163.8, 188.0); nausea (326.4/210.3, 207.8); hyperkalaemia (234.6/161.9, 158.3); headache (214.2/119.1, 118.7); somnolence (204.0/40.9, 98.9); and back pain (112.2/87.5, 39.6). As can be seen, IR/1,000 pt-yrs for all TEAEs in those taking difelikefalin decreased in the placebo-controlled + OLE periods compared to the initial 12-week trial period.10
TEAEs were mostly mild to moderate in severity and the IR/1,000 pt-yrs for the most common TEAEs and serious TEAEs did not increase with longer-term exposure. The authors concluded that difelikefalin was well tolerated with an acceptable long-term safety profile.10
Overall, considering both long-term efficacy and safety data, the authors concluded that “These findings suggest that difelikefalin may help to address the unmet need for treatments that are well tolerated and efficacious over the long term for moderate-to-severe CKD-aP in patients undergoing heamodialysis.”10
Insights into Potential Real-World Findings with Difelikefalin Use
While the KALM-1 and KALM-2 trials provided very useful clinical information regarding difelikefalin efficacy and safety, as with any drug, open-label studies can aid in providing additional, more ‘real world’ data. In his talk, Weiner presented a Phase III, open-label, multicentre study that evaluated not only intravenous difelikefalin safety and efficacy in patients with moderate-to-severe CKD-aP undergoing haemodialysis, but also QoL issues such as sleep quality and self-confidence.11
Eligible patients were 18−85 years of age who had been undergoing haemodialysis at least three times per week for at least 3 months. In total, 222 patients received 0.5 mg/kg intravenous difelikefalin three times per week for 12 weeks, and 197 (88.7%) completed the study. Most patients came from the USA (n=203), with others coming from Hungary (n=12), the Czech Republic (n=4), and Poland (n=3) 11
In the week prior to difelikefalin administration, patients needed to have a score of ≥5 on the WI-NRS.4,12 For this study, a ≥3-point and ≥4-point change from baseline was assessed, as well as noting those who attained complete resolution, defined as ≥75% of weekly mean WI-NRS scores equal to 0 or 1. Weiner highlighted how the ≥3-point change is “what has been validated as clinically meaningful in this population,” with the ≥4-point change being “what’s commonly used by the U.S. Food and Drug Administration (FDA) in studies with dermatologic indications.”11
Post-hoc scores on a study-specific Sleep Quality Questionnaire, with a range from 0 (did not interfere) to 10 (completely interfered), were also assessed using these endpoints. Other pre-defined outcomes investigated itch-related QoL with the 5-D Itch Scale and the Skindex-10 Scale. Domains on the EuroQol psoriasis-specific questionnaire regarding skin irritation and self-confidence were also used to assess these issues.21
Weiner reported that the patients in this study were “a fairly typical dialysis population, albeit a little bit younger than many that we see in the USA.” Patients’ mean (SD) age was 58.1 (12.8) years, and slightly more were male (54.5%). They weighed on average 86.6 (23.5) kg, had been receiving haemodialysis for a mean of 5.4 (4.4) years, and recalled that they had experienced CKD-aP for a mean of 3.9 (3.3) years.11 This latter finding was similar to what was reported in the KALM-1 and KALM-2 studies18,19 and indicates, highlighted Weiner, that “this is not a new problem for many of the patients in the trial.”
To gain an understanding of this cohort prior to difelikefalin initiation, baseline data were taken regarding health parameters and current CKD-aP treatment. CKD-associated aetiologies included hypertension (60.8%) and glomerulonephritis (5.0%). While 60.4% of patients had a medical history of diabetes, only 49.5% had CKD related to this. At baseline, the mean (SD) for bilirubin was 8.8 (4.2) mmol/L, for calcium was 2.2 (0.2) mmol/L, and for phosphate was 1.9 (0.6) mmol/L. These laboratory data were interesting, noted Weiner, as the normal bilirubin levels indicated there was no liver indication for pruritus, and that while phosphate levels were slightly above normal, which he discussed could be associated with hyperparathyroidism or hyperphosphataemia, overall, there were “no glaring laboratory abnormalities that could explain pruritus in these individuals.” Of the patients in this cohort, 32% were using an ‘anti-itch’ medication at baseline, predominantly diphenhydramine (22.1%) or hydroxyzine (6.3%).11
At baseline, mean (SD) WI-NRS score was 7.6 (1.3), Sleep Quality Questionnaire score was 6.6 (2.2), 5-D Itch Scale total score was 17.1 (3.5), and Skindex-10 Scale score was 32.9 (14.3). Overall by Week 12, discussed Weiner, “people indicated that they were responding after treatment with difelikefalin.” Most patients achieved the primary endpoints of a ≥3-point and ≥4-point improvement in WI-NRS and Sleep Quality scores with 29.4% and 19.1%, respectively, showing complete resolution of symptoms on these scales. Also noted were mean changes from baseline in the 5-D Itch total score of -7.1 (SD 4.27; n=192), and in the Skindex-10 total score of -21.0 (SD 15.59) (Figure 3). Both of these score changes represent a clinically meaningful improvement.11
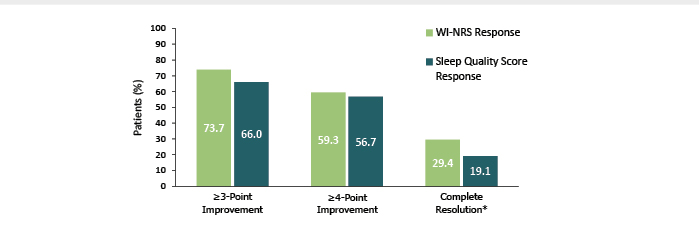
Figure 3: Percentage of patients who achieved a ≥3- or ≥4-point improvement in WI-NRS response and Sleep Quality Score response and who achieved complete resolution* at Week 12 (n=194).11
*≥75% of weekly mean WI-NRS scores equal to 0 or 1 or all Sleep Quality scores equal to 0. During the 1-week run-in period and at baseline 2.7% of patients had all Sleep Quality scores equal to 0.
WI-NRS: Worst Itching Intensity Numerical Rating Scale.
Another way of addressing how CKD-aP affects an individual is investigating levels of skin irritation, as examined with the EuroQol psoriasis-specific questionnaire. This measure revealed that while at baseline, 91% of 190 patients reported at least moderate problems with skin irritation, after 12 weeks’ administration of difelikefalin 28.9% reported ‘no problems’.11 A further QoL factor for people with CKD-aP, as with many skin conditions, is self-confidence, reported on the EuroQol psoriasis-specific questionnaire as at least ‘slight’ by 36.7% at baseline, falling to 26.8% after 12 weeks.11
Similar to the KALM-1 and KALM-2 studies,10,18,19 the most frequently reported TEAEs (≥4% of patients) were diarrhoea (5.0%), nausea (4.5%), and hyperkalaemia (4.1%), with overall (n=222) 64.4% of patients reporting a TEAE. While 20.3% of these were classed as serious, no serious TEAE was related to difelikefalin. Of those with a TEAE, 6.3% led to treatment discontinuation.11
Weiner concluded that for these patients, difelikefalin “was effective at reducing self-reported itch intensity and was associated with improved sleep quality and itch-related QoL.” Difelikefalin was also generally well tolerated with an acceptable safety profile.
These findings confirm those of the previous Phase III placebo-controlled studies.18,19 Importantly, the advantage of this open-label trial is that it provides an insight into the potential real-world effectiveness of difelikefalin. “We practice medicine openly,” said Weiner, “and this gives us an idea of what to expect with these agents, going forward.”11
Conclusions
CKD-aP can affect many patients undergoing haemodialysis, more than is currently appreciated, and sometimes can be a life-disrupting condition. However, it is under-reported and there is a need for new CKD-aP-specific treatments. By targeting peripheral KORs, difelikefalin has been shown an effective treatment for people with moderate-to-severe CKD-aP, with a favourable safety profile and impact on QoL factors such as sleep.

![EMJ Nephrology 9 [Supplement 3] 2021 Feature Image](https://www.emjreviews.com/wp-content/uploads/2021/08/EMJ-Nephrology-9-Supplement-3-2021-Feature-Image-940x563.jpg)






