BACKGROUND AND AIMS
Rituximab (RTX) is used worldwide for the treatment of glomerulonephritis (GN), including membranous glomerulopathy (MN), minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), and ANCA-mediated vasculitis.1-5 However, its role in IgA nephropathy (IgAN), lupus nephritis (LN), and C3 glomerulonephritis (C3GN) is still not clear.6-9 The authors hypothesised that RTX is used by Kuwait nephrologists for all immune-mediated glomerular disease as salvage therapy to induce remission, despite a lack of established indication for use; that is, off-label use. This study reviewed and assessed the indications for use of RTX and compared these to the available established literature and review outcomes.
MATERIALS AND METHODS
Data for patients with GN aged ≥18 years who had received RTX treatment by a nephrologist were collected from three public hospitals in Kuwait between January 2014 and January 2018. The indication to administer RTX was solely determined by the nephrologist in charge.
Patients were separated based on their original GN subtype. For analysis purposes, MN and MCD were categorised into a disease group with data supporting RTX use, based on the literature available. In comparison, the remaining cases were categorised into a group with insufficient data to support RTX use (i.e., an off-label group), which included FSGS, LN, IgA nephropathy, IgG4-related disease, C3 GN, and membranoproliferative GN. Clinical remission was assessed 6 months after receiving RTX.
Statistical analysis was performed using Stata version 16 (StataCorp, College Station, Texas, USA). Statistical tests comparing categorical variables were performed as chi-squared tests. A logistic regression model was used to assess the probability of remission.
RESULTS
A total of 67 cases of glomerular diseases were reviewed. Three patients were excluded because of missing outcome data, and three for refusal of biopsy (two with LN, and one with presumed MCD). A total of 61 cases were included in the final analysis (Table 1). No major side-effects were reported. RTX was an add-on therapy in most cases.
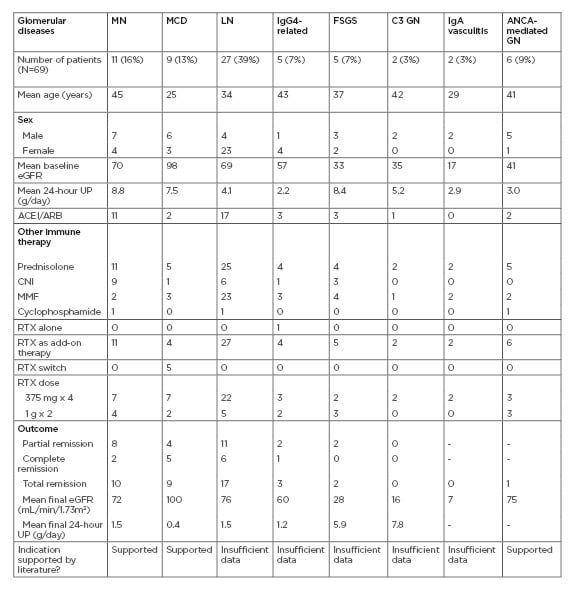
Table 1: Baseline characteristics of patients studied, and outcomes following treatment with rituximab.
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CNI: calcineurin inhibitor; eGFR: estimated glomerular filtration rate; FSGS: focal segmental glomerulosclerosis; GN: glomerulonephritis; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; MMF: mycophenolate mofetil; MN: membranous nephropathy; RTX: rituximab; UP: urinary protein.
The remission rate was 95% in diseases with data supporting the use of RTX (MCD and MN), compared to 56% in the off-label group (p value=0.002). The crude odds ratio for remission in the diseases supporting the use of RTX was 15 (confidence interval: 1.2–152), while the crude odds ratio for remission in the off-label group was 0.07 (confidence interval: 0.008–0.55; p value=0.012). For the MN patients, anti-PLA2R antibody testing was not performed on serum samples, but was positive in all of the biopsies. In the off-label group, patients with LN had a reasonable mean initial estimated glomerular filtration rate.
All five patients with Class III LN achieved remission with RTX, and 11 of the 21 patients with Class IV LN achieved remission. There was one further patient with Class V LN, who achieved remission. The patients with FSGS had a low mean initial estimated glomerular filtration rate that did not improve, and only two out of the five patients showed partial resolution of proteinuria. Not included in the table is a case of membranoproliferative GN that showed no response, and a case of IgAN that showed partial remission.
Independently, eight cases of vasculitis were reviewed (six cases of pauci-immune ANCA-related vasculitis and two cases of IgA vasculitis) that received RTX during the same period but were not included in the above analysis. Remission was achieved in only two of the patients with pauci-immune vasculitis, and in none of the patients with IgA vasculitis.
CONCLUSION
These data are in line with the literature supporting RTX use in resistant MCD and MN. However, RTX off-label use in other GN subtypes is still not clear, despite some successes in LN and IgG4-related disease. Because of the small number of patients with vasculitis, this study cannot comment on the effectiveness of RTX in this group. Overall, this study provided significant insight on RTX use for GN in Kuwait.








