BACKGROUND
Eosinophilic granulomatosis with polyangiitis (EGPA) is a small vessel vasculitis characterised by the presence of tissue eosinophilia, necrotising vasculitis, and granulomatous inflammation.1 Typically, a prodromal asthmatic phase, leads to an eosinophilic stage, which can evolve to include the presence of vasculitis with renal manifestations.2,3 In the recent randomised, placebo-controlled MIRRA trial for relapsing and refractory EGPA, adjuvant therapy with the anti-IL-5 monoclonal antibody, mepolizumab (MEPO), at 300 mg subcutaneously (SC) monthly, resulted in a longer remission period, reduced steroid exposure, and reduced relapse rates.4,5
AIMS
The aim of this study was to analyse the response and outcome for EGPA patients who received MEPO monthly for a minimum of 52 weeks, with particular focus on the steroid minimisation benefits. This retrospective, descriptive study analysed 13 EGPA patients, who received 100 mg SC monthly MEPO therapy under the eosinophilic asthma care pathway. Time points of assessment included MEPO commencement (M0) and 12 months (M12).
RESULTS
This study demonstrated that anti-IL-5 therapy serves as a favourable model with steroid minimisation, improvement in the Asthma Control Questionnaire (ACQ), reduction in the Birmingham Vasculitis Activity Score (BVAS) and eosinophil counts at 100 mg SC dosage (Table 1). Anti-neutrophil cytoplasm antibody positive serology normalised in all four patients, independent of subtype. MEPO was well tolerated, and demonstrated considerable clinical benefit, with 12 patients (92.3%) continuing anti-IL-5 therapy beyond 12 months.6,7 One patient had MEPO switched to rituximab to treat both EGPA and new onset rheumatoid arthritis. Adjuvant therapy with conventional immunosuppressants was well tolerated and renal function was preserved.
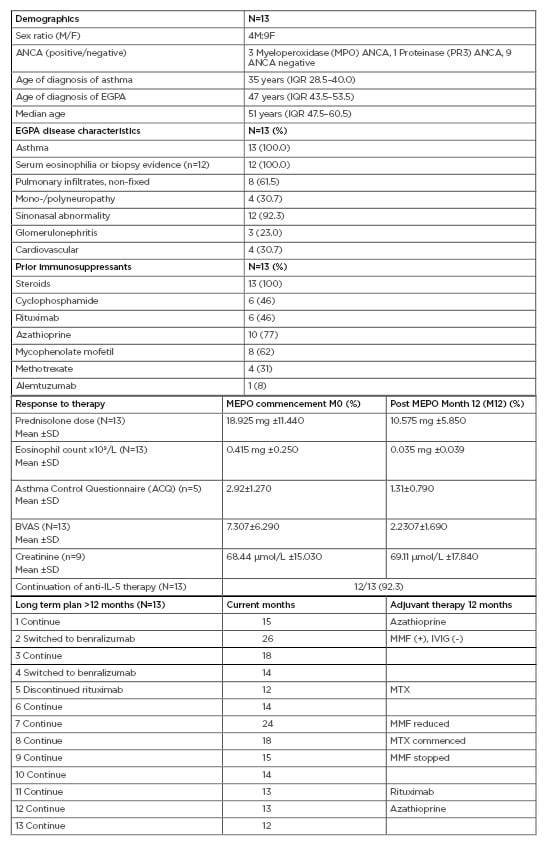
Table 1: Eosinophilic granulomatosis with polyangiitis patients receiving mepolizumab therapy for 1 year (100 mg subcutaneously).
ACQ: Asthma Control Questionnaire; ANCA: Anti-neutrophil cytoplasm antibodies; BVAS: Birmingham Vasculitis Activity Score; EGPA: eosinophilic granulomatosis with polyangiitis; F: female; IQR: interquartile range; IVIG: intravenous immunoglobulin; M: male; MMF: mycophenolate mofetil, MTX: methotrexate; SD: standard deviation.
CONCLUSION
The relapsing nature of EGPA places a potential dependency of therapy on steroids for asthmatic and vasculitic flares.8 This underscores the importance of pathway targeted biologic therapy to minimise steroid exposure, prevent tissue damage accrual, and ensure early response to treatment.9,10








