BACKGROUND
While cardiac tumours have been documented since the 1500s, first featuring in Italian anatomist and surgeon Colombo’s ‘De re anatomica’ (‘On Things Anatomical’)1,2, it was not until 1952 that Banhson and Newman performed the first successful open surgical excision of a primary cardiac tumour, followed by Crafoord et al. in 1954 using a cardiopulmonary bypass.3,4
The incidence of primary cardiac tumours discovered at autopsy is around 0.02%, with 75% of these being histologically benign.5 Cardiac haemangiomas are extremely rare, accounting for approximately 1–2% of all primary cardiac tumours. They are benign vascular tumours that can occur in any chamber of the heart, predominantly on the right side, with only 7% of cases arising from the left atrium, which was seen in this case.6,7 They may occur in any of the three cardiac layers: the endocardium, myocardium, and pericardium. They are rarely found in the intra-atrial septum, intraventricular septum, or on the valves.
Cardiac haemangiomas are characterised microscopically by benign proliferative endothelial cells lining blood vessels, with increased vascularisation.6 They are classified into three subgroups, according to histological appearances: cavernous (composed of vessels with wide lumens), capillary, and arteriovenous malformations (angiodysplasias).8,9
As patients are commonly asymptomatic, cardiac haemangiomas are often discovered incidentally on imaging or revealed during autopsy. However, depending on the anatomical location of the tumour, patients can present with breathlessness, congestive heart failure, chest pain, syncope, pericardial effusion, and valve stenosis. Although benign histologically, if left untreated, cardiac haemangiomas can rupture or give rise to conductive and haemodynamic abnormalities, resulting in significant morbidity and mortality.10 Therefore, surgical resection is the treatment of choice. There are case reports of conservative management approaches without obvious major complications in adults where resection was deemed too high-risk.11,12
CASE PRESENTATION
A 69-year-old female was referred by her general practitioner to an elderly care clinic with worsening exertional dyspnoea and fatigue for 3 months. She also reported an unexplained 3 kg weight loss in the same period, with no change in her lifestyle to account for this. Her exercise tolerance was unlimited on flat ground; however, she could only complete a flight of stairs at a slow pace, being limited by fatigue and breathlessness.
Her past medical history included chronic obstructive pulmonary disease, breast fibroadenoma, kidney stones, solitary lipoma removal, and a recent diagnosis of paroxysmal atrial fibrillation. She was an ex-smoker (20 pack-years) and had a family history of diabetes and stroke. Her medication history included lansoprazole, inhaled tiotropium, inhaled budesonide and formoterol fumarate, inhaled salbutamol, and flecainide. Physical examination was normal, with a blood pressure of 137/80 mmHg and a regular heart rate of 67 beats per minute.
INVESTIGATIONS
Baseline investigations demonstrated normal renal function, full blood count, C-reactive protein, plasma viscosity, bone profile, CA125, liver function, thyroid function, vitamin B12, and folate. Her ferritin was only mildly raised at 167 μg/L. Chest X-ray showed emphysematous changes.
A CT scan of the chest, abdomen, and pelvis to screen for malignancy demonstrated a prominent atrial appendage, with a possible filling defect (Figure 1A). There was no evidence of lymphadenopathy. Given the recent diagnosis of atrial fibrillation, the mass was initially considered to be a thrombus. Transoesophageal echocardiography (Figure 1C) did not clearly show the left atrial appendage (LAA) as it appeared either compressed externally or largely replaced/full of a mass. The mass appeared heterogeneous and larger than her CT at 39×60 mm. There was no obvious invasion into surrounding structures and one view showed flow within. There were no other structural abnormalities.
She went on to have a cardiac MRI (cMRI; Figure 1D). This showed a mass originating from within the LAA and measuring approximately 50×35 mm, with no invasion beyond this. There was homogeneous signal on T1-weighted sequences, some heterogeneity on T2-weighted sequences, and hyperintensity on T2 short tau inversion recovery (fat saturation) sequences (Figure 2). Real-time imaging during gadolinium administration suggested some perfusion but not extremely brisk. In the early phase following gadolinium there was heterogeneity, with some uptake and other areas which appeared avascular. There was no significant compression of other cardiac or vascular structures. The mass most likely represented a LAA myxoma with possible thrombus.
Given her progressive dyspnoea and the potential risk of life-threatening complications such as embolism and arrhythmia, she was referred for surgical excision of the LAA mass.
She subsequently underwent coronary angiography as a work-up for surgery. This demonstrated normal coronary arteries and a vascular blush through the tumour (Figure 1B).
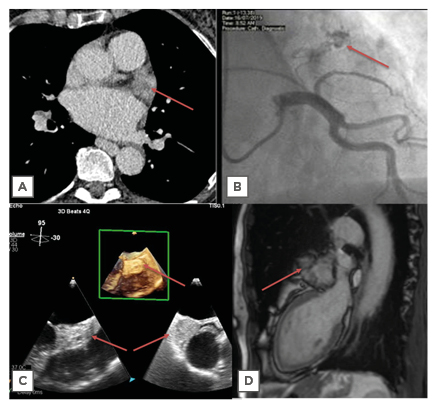
Figure 1: Imaging modalities, with cardiac mass highlighted by red arrow. A) Transverse section on CT; B) right anterior oblique cranial view on angiography showing neovascular formation supplying haemangioma; C) transoesophageal echocardiography with 3D imaging showing a left atrium mass; D) sagittal section on cardiac MRI.
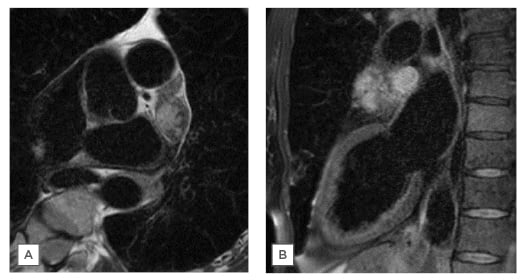
Figure 2: T2 STIR MRI images with fat suppression. A) Transverse section; B) sagittal section.
STIR: short tau inversion recovery.
TREATMENT
She underwent surgical resection, and histology demonstrated sections of atrium containing a well-circumscribed lesion, comprising multiple thick- and thin-walled blood-filled vascular channels, with foci of papillary endothelial hyperplasia. There was no evidence of atypia or necrosis. The histological findings were characteristic of a benign cavernous haemangioma.
Following surgery, she was repatriated to the local district general hospital for post-operative rehabilitation, which was complicated by hospital-acquired pneumonia requiring intravenous antibiotics and pulmonary oedema requiring treatment with intravenous administration of furosemide.
In a 3-month follow-up clinic she reported a reduction in peripheral limb oedema but ongoing breathlessness and chest tightness. This was attributed to resolving pleural effusions and chronic obstructive pulmonary disease. Unfortunately, the patient developed recurrent chest infections and is under the respiratory team for further follow-up. A 1-year follow-up transthoracic echocardiogram demonstrated no recurrence of the haemangioma.
DISCUSSION
Cardiac haemangiomas are rare benign cardiac tumours that are histologically like that of their extracardiac counterparts. They develop from proliferation of endothelial cells and pericytes. As mentioned previously, they can be divided into three subgroups: cavernous, capillary, and arteriovenous malformations. The histological appearances of the capillary subgroup demonstrate a myxoid matrix with capillary-sized vessels, endothelial cells, pericytes, and fibroblasts. The cavernous pattern consists of large, dilated vascular spaces with either thick or thin walls. Lastly, the arteriovenous pattern is characterised by heterogeneous dysplastic vessel types with muscularised vessels or irregular, thickened wall diameter and, on occasion, containing fibrous and fat tissue. However, cardiac haemangiomas may be a combination of the three patterns. Immunohistochemistry can reveal expression of typical endothelial markers including CD31, CD34, and factor VIII.
Cardiac haemangiomas, including cavernous haemangiomas, are generally sporadic in nature and can be found in all areas of the heart but rarely in the epicardium or valves.9,13 The authors’ case demonstrated a cardiac haemangioma in the LAA, which is very rare. Han Y et al.14 reviewed 56 cases and found that most cardiac haemangiomas were found on the right side of the heart, with 7% seen in the left atrium. Cardiac cavernous haemangiomas are slightly more commonly seen in females and can occur at any age.10
There are several cardiac imaging modalities available for the work-up of a suspected cardiac tumour, each with varying benefits and limitations (Table 1). Transthoracic echocardiography is an effective, non-invasive investigation with a sensitivity of 93% and is particularly useful in diagnosing tumours within the ventricles. Transoesophageal echocardiography provides a slightly more effective diagnostic modality, particularly for tumours within the atria (97% sensitivity).13 Echocardiography in general can be utilised to assess size, morphology, attachments to adjacent structures, extension, and haemodynamic effects and can improve intracavity definition, assess vascularity, and exclude thrombus. CT is established in its use to assess chest and lung tissue and corresponding vascular structures, assess masses involving the coronaries, and exclude coronary artery disease and calcified masses. PET can be useful for differentiating between benign and malignant lesions, staging malignancies, optimising biopsy location, and planning radiotherapy in certain circumstances. cMRI is considered the gold standard imaging modality for assessing possible cardiac tumours. It is non-invasive and produces high-resolution images without using ionising radiation.15
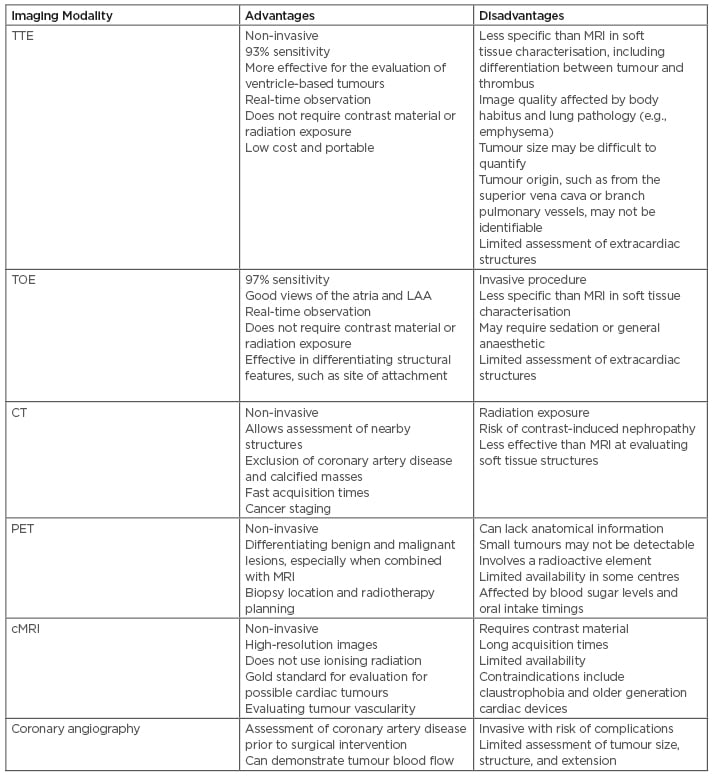
Table 1: Advantages and disadvantages of different imaging modalities for assessment of a suspected cardiac tumour.
cMRI: cardiac MRI; LAA: left atrial appendage; PET: positron emission tomography; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
cMRI can effectively assess morphology, dimensions, location, extension, and infiltration into adjacent tissue, and characterise histopathology.16 Coronary angiography is useful pre-operatively to assess for coronary artery disease as coronary artery bypass grafting can be undertaken at the same time as resecting the tumour. It can also demonstrate the blood supply to the tumour and reveal the characteristic vascular blush, which is associated with many cardiac tumours. Interestingly, the vascular blush seen with most cardiac tumours, including haemangiomas, is not typically associated with the cavernous subtype because of the large vascular spaces that promote very slow flow.11 However, coronary angiography did reveal this finding in the authors’ case.
Tumour resection can occur under extracorporeal membrane oxygenation installed using aortic and bicaval venous cannulation, with concomitant valve surgery if required. Post-operative complications may include arrhythmia, major bleeding into the chest, renal dysfunction, and wound infection. As a consequence of the low incidence of haemangiomas, large cohort published studies are rare in regard to post-operative survival; however, one article reported survival following resection similar to a standard population but recommended close follow-up with annual echocardiography for the first 4 years minimally.17
LEARNING POINTS
Cardiac cavernous haemangiomas are rare benign tumours more commonly seen in the right heart; this case demonstrated a LAA cavernous haemangioma, which was unusual. Surgical resection is the recommended approach to management to relieve symptoms and reduce the risk of life-threatening complications, including tumour rupture, embolism, arrhythmia, and haemodynamic compromise. There are a number of imaging modalities to assess cardiac tumours. cMRI remains the gold standard for assessment because of its non-invasive nature and production of high-resolution images.







