Abstract
There is mounting evidence to support the impact of sarcopenia on the prognosis of a wide range of clinical conditions. This review examines the literature on the effect of body composition measures, including sarcopenia, on outcomes in patients with hepatocellular carcinoma (HCC). Available studies support the adverse impact that sarcopenia has on overall survival, response to different treatment modalities, and tumour recurrence. Some studies have identified visceral fat deposition as a negative prognostic sign, and the incorporation of body composition measures into current HCC staging schemes have been shown to improve prognostic accuracy. On the other hand, there is a paucity of studies assessing nutritional interventions in HCC and further trials are needed to inform evidence-based practice.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of solid tumour mortality worldwide, with over half a million new cases diagnosed annually around the world.1 In Europe, HCC accounts for around 47,000 deaths a year, with mortality rates expected to rise over the next decade.2 The prognosis in HCC is poor, with a 5-year survival rate of approximately 10%.3 In >90% of cases, HCC develops in patients with liver cirrhosis, most commonly due to infection with hepatitis B or C virus.4 Other aetiological factors for the development of HCC include chronic alcohol consumption and nonalcoholic fatty liver disease (NAFLD).4
The Barcelona Clinic Liver Cancer (BCLC) staging system incorporates information on tumour burden, underlying liver function, and clinical features (e.g., performance status) to guide therapeutic decision-making and prognostication in HCC.5 Treatment for HCC comprises both surgical and nonsurgical techniques. Hepatic resection or liver transplantation is reserved for early stage disease.5 More advanced cases of HCC are considered for nonsurgical treatment modalities, including radiofrequency ablation (RFA), transarterial chemoembolisation (TACE), and sorafenib.5
Although it remains one of the best HCC staging systems, BCLC has some shortcomings, particularly with regard to prognostication in the highly heterogenous patient population with intermediate-stage HCC (Stage B).6 There is increasing research interest into the pathological, genetic, and clinical factors that may improve HCC prognostication and guide appropriate treatment choice.7 In particular, the addition of clinical factors, such as sarcopenia and visceral fat deposition derived from computed tomography (CT) scanning, have been shown to improve prognostic accuracy compared with the BCLC staging system alone.8 Therefore, it is important that the effect of body composition variables on outcome in HCC is delineated and the role of clinical nutrition in influencing response to surgical and medical treatments is adequately explored.
This review describes the existing knowledge on the effect of sarcopenia on outcomes in HCC and will also present the role of other body composition changes. Finally, the available studies evaluating nutritional interventions will be summarised to guide practical recommendations for optimisation of nutritional status in patients with HCC.
METHODS
Relevant studies were identified by searching the medical databases Embase and Medline (January 1996–March 2018). Additional searches were carried out using Google Scholar and Clinical Key® (Elsevier, Amsterdam, Netherlands) to identify other published data on the subject. Two separate searches were conducted; the first included the following search terms: “Hepatocelluar carcinoma” AND (“Sarcopenia” OR “Body composition”). The second search included the following terms: “Hepatocellular carcinoma” AND (“Nutrition support” OR “Dietary supplements” OR “Enteral nutrition” OR “Nutrition intervention”). A total of 139 and 209 studies were generated from the two searches, respectively, and relevant articles were selected following screening of titles, keywords, and abstracts.
All studies, either interventional or observational, that evaluated body composition measures or nutritional interventions in HCC were included. Studies evaluating sarcopenia were required to quantify skeletal muscle mass by CT or magnetic resonance imaging (MRI) scanning, which are considered the gold standard techniques for estimating muscle mass.9 These methods overcome the difficulties associated with accurate nutritional assessment in patients with ascites and oedema related to underlying liver disease. Eighteen papers were selected for inclusion in this review; Tables 1, 2, and 3 summarise the chosen studies, including study design, participants, and findings. Excluded articles were those with no relevance to the topic, general review articles, and conference abstracts.
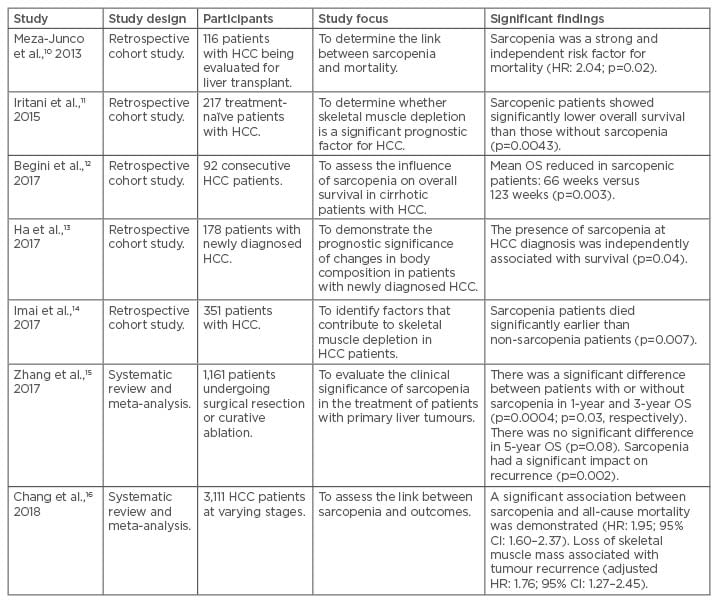
Table 1: Summary of studies assessing the impact of sarcopenia on hepatocellular carcinoma.
CI: confidence interval; HCC: hepatocellular carcinoma; HR: hazard ratio; OS: overall survival.
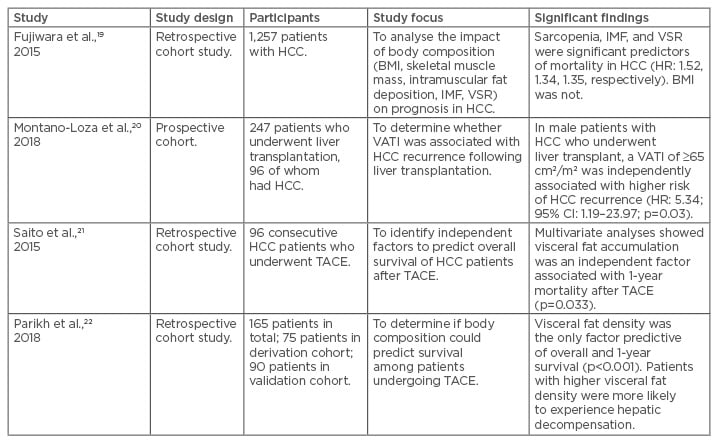
Table 2: Summary of studies evaluating the effect of adipose tissue distribution on hepatocellular carcinoma.
CI: confidence interval; HCC: hepatocellular carcinoma; HR: hazard ratio; IMF: intramuscular fat deposition; TACE: transarterial chemoembolisation; VATI: visceral adipose tissue index; VSR: visceral-to-subcutaneous adipose tissue area ratio.
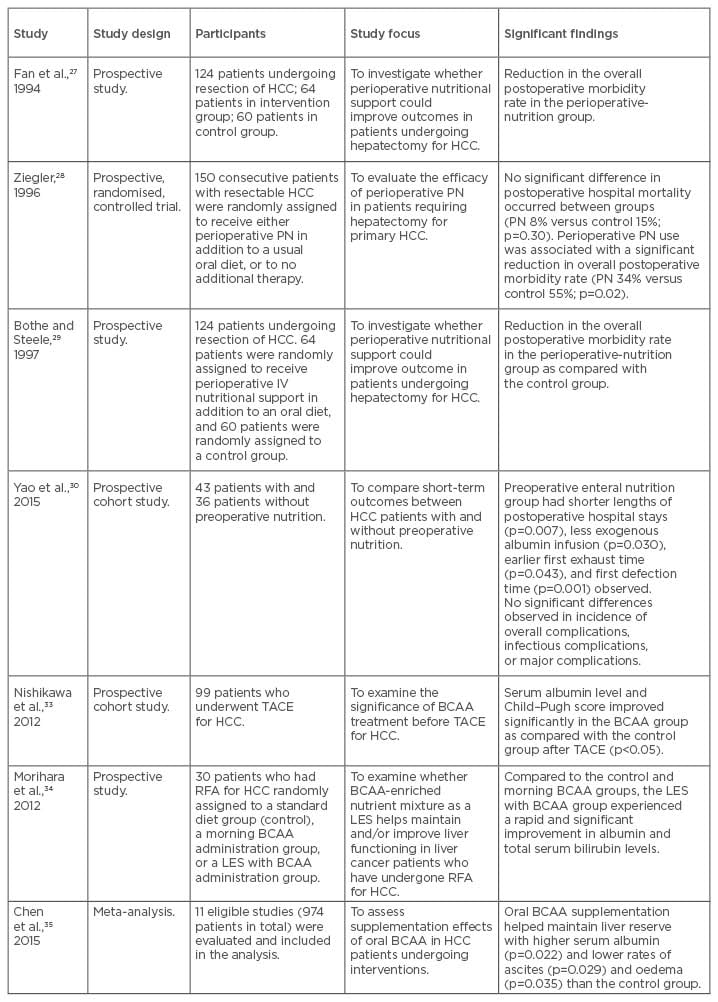
Table 3: Summary of studies evaluating nutritional or exercise interventions in hepatocellular carcinoma.
BCAA: branched-chain amino acid; HCC: hepatocellular carcinoma; IV: intravenous; LES: late evening snack; PN: parenteral nutrition; RFA: radiofrequency ablation; TACE: transarterial chemoembolisation.
RESULTS
The Effect of Sarcopenia on Hepatocellular Carcinoma
Sarcopenia is associated with ageing and chronic illness and is defined by the European Working Group on Sarcopenia in Older People (EWGSOP) as the loss of muscle mass and strength.9 Most of the available studies on sarcopenia and HCC, however, are retrospective and did not include measurements for muscle strength at the data collection stage. Therefore, for the purposes of this review, sarcopenia is used interchangeably with the skeletal muscle index or the cross-sectional area of muscles at the third lumbar vertebral level on CT scanning, measured in cm2/m2.
Meza-Junco et al.10 found that sarcopenia was an independent risk factor for mortality in 116 patients with HCC undergoing evaluation for liver transplantation (hazard ratio [HR]: 2.04; p=0.02). Later, Iritani et al.11 evaluated 217 treatment-naïve HCC patients of any stage and found that those with sarcopenia had a significantly reduced overall survival (p=0.0043). In 2017, two further retrospective analyses12,13 identified sarcopenia as a significant risk factor for overall survival in 92 and 178 newly diagnosed HCC patients, respectively. A further study in 201714 showed that patients with sarcopenia died significantly earlier than nonsarcopenic patients (p=0.007); however, this difference was not measured following adjustment for age, sex, tumour size, tumour number, and the degree of portal vein invasion.
Two recent meta-analyses15,16 corroborated the above findings. Zhang et al.15 evaluated eight retrospective studies that included 1,161 patients undergoing mostly surgical interventions or curative RFA. They showed that HCC patients with sarcopenia had a poorer prognosis with respect to the 1-year and 3-year overall survival, 5-year disease-free survival, incidence of recurrence, and post-treatment overall complications. Chang et al.16 investigated 13 studies comprising 3,111 patients with HCC of varying stages. A significant association between sarcopenia and all-cause mortality was demonstrated (HR: 1.95; 95% confidence interval: 1.60–2.37). Similarly, loss of skeletal muscle mass was associated with tumour recurrence (adjusted HR: 1.76; 95% confidence interval: 1.27–2.45). In both analyses, the researchers cited the limitations of the included studies, including the absence of a consistent definition for sarcopenia in terms of cm2/m2, low patient numbers, and a lack of any measures for muscle function.
Overall, the available studies demonstrate the adverse impact that sarcopenia has on the prognosis of HCC of varying stages, both prior to and following medical and surgical treatments. Sarcopenia also increases the risk of tumour recurrence following curative treatment. However, the studies are hampered by their mainly retrospective designs, and further prospective studies incorporating measurements of muscle strength and physical function are needed to determine whether these better predict the outcome than use of muscle mass alone. Finally, the available research does not address the underlying mechanisms by which sarcopenia worsens outcomes in HCC. An increased understanding of these mechanisms of muscle depletion would engender improved interventions and treatment strategies.
The Effect of Adipose Tissue Distribution on Hepatocellular Carcinoma
NAFLD has more recently been recognised as another important aetiological factor for the development of liver cirrhosis and HCC.17 Liver cirrhosis in this context can lead to further skeletal muscle loss and gain of adipose tissue, culminating in sarcopenic obesity.18 NAFLD is also one of the main risk factors for HCC development in the absence of established liver cirrhosis.17
A handful of studies have demonstrated the importance of adipose tissue distribution on outcomes in HCC. Fujiwara et al.19 examined the CT scans of 1,257 patients with HCC of differing stages and with differing treatment plans. Multivariate analysis revealed that, in addition to low skeletal muscle index, both high visceral-to-subcutaneous adiposity ratio and low mean muscle attenuation (increased intramuscular fat deposition) were significantly associated with mortality (HR: 1.52, p=0.001; HR: 1.35, p=0.005; and HR: 1.34, p=0.02, respectively). In liver transplantation, one study found that male patients with cirrhosis and increased visceral adiposity had higher rates of HCC recurrence.20 Similarly, two studies21,22 evaluating body composition measures in HCC patients undergoing TACE found that patients with higher visceral fat density and area were at an increased risk of death at 1 year. Moreover, patients with higher visceral fat density were more likely to experience hepatic decompensation after TACE (p<0.001).
The reasons for the negative impact of visceral and intramuscular adipose tissue deposition on HCC outcomes are not fully understood. In sarcopenic obesity, muscle depletion is characterised by both a reduction in muscle size and an increased proportion of inter and intramuscular fat deposition, named myosteatosis.23 Myosteatosis is associated with increasing insulin resistance and decreased strength and mobility.24 Visceral fat accumulation increases the levels of proinflammatory adipokines, such as tumour necrosis factor-alpha, interleukin-6, and monocyte chemoattractant protein-1, and decreases the level of the anti-inflammatory adipokine adiponectin.25 Excess visceral fat may also contribute to portal hypertension because free fatty acids and adipokines released from visceral fat flow directly into the liver via the portal vein.26 It is clear the exact mechanisms underlying the interaction of visceral adiposity, liver function, and prognosis in HCC are highly complex and require further investigation.
Nutritional Interventions to Improve Outcome in Hepatocellular Carcinoma
Interventional studies addressing the effect of nutritional therapy in HCC patients are few. The benefits of perioperative nutrition support on outcomes were evaluated in a series of studies in the mid-1990s.27-29 These demonstrated that perioperative nutrition support for 14 days with a parenteral nutrition (PN) solution rich in branched-chain amino acids (BCAA) resulted in a reduction of morbidity, diuretic use for ascites control, weight loss post-hepatectomy, and liver function, as measured by indocyanine green clearance. A more recent cohort study30 compared patients who had received enteral nutrition 3 days prior to hepatic resection with a medium-chain triglyceride-rich formula with those who had not. They found a shorter length of hospital stay and less exogenous albumin use in the group that had received enteral nutrition. There were no significant differences seen in the incidence of overall complications, infectious complications, or major complications. Additional data are needed to delineate further the relationship between pre and postoperative nutrition support and outcomes following HCC resection.
The benefits of BCAA supplementation and a late evening snack in patients with liver cirrhosis alone have been explored recently, with studies suggesting that these strategies hold promise in reversing anabolic resistance and sarcopenia, improving quality of life.31,32 In HCC, one study33 evaluated the effects of BCAA supplementation in 40 patients 1 month prior to TACE and compared these to 59 control patients on a normal diet. They found that the BCAA group had significantly improved albumin levels and Child–Pugh scores at 3 and 6 months post-TACE (p<0.05). Similarly, the benefits that a late evening snack containing BCAA had in improving Child-Pugh scores were demonstrated by Morihara et al.34 Results from a meta-analysis of 11 studies35 showed that BCAA supplementation conferred a 3-year mortality benefit, higher serum albumin levels, and lower rates of ascites. The 1-year mortality and HCC recurrence rates were not improved by BCAA supplementation, however, and many of the included studies were retrospective and had small sample sizes. All participants in this study had liver cirrhosis and therefore the benefit of BCAA supplements in HCC patients without cirrhosis is unknown.
DISCUSSION
There is much evidence to support the deleterious effects of sarcopenia on a wide range of clinical conditions. This is no different in HCC and the available studies demonstrate the adverse impact of sarcopenia on overall survival, response to different treatment modalities, and tumour recurrence following curative resection. In addition to sarcopenia, some studies have identified visceral fat deposition as a negative prognostic sign and the development of sarcopenic obesity appears to drive poorer responses to treatment and reduces survival rates.
Nonetheless, the available body composition data in HCC are limited to mainly retrospective studies, making conclusions on causality difficult. For example, it is unclear whether sarcopenia is driven by the catabolic effects of liver cirrhosis, neoplasia, or a combination of the two. Increasingly, HCC in NAFLD23 occurs in the absence of cirrhosis, and the mechanisms by which intrahepatic, visceral, and intramuscular fat deposition causes neoplastic effects are yet to be established. An increased understanding of these mechanisms would help inform practical evidence-based guidance on nutrition support in HCC, and this review has highlighted the distinct paucity of nutrition intervention studies in this field. Inferences on the optimum nutrition management from guidance on liver cirrhosis and cancers may not be applicable to HCC patients given the unique cell biology of the condition and complex interplay with liver function and gut microbiota.
Future studies, therefore, should focus on the mechanisms by which sarcopenia and adipose tissue distribution effect HCC prognosis. Whether these measures adversely impact survival by undermining liver function or promoting tumour invasion and metastasis is unknown. An improved understanding of the relationship between body composition, response to therapy, and prognosis will enable the formulation of a tailored management strategy. Nutritional intervention studies in HCC are warranted to investigate the effect of manipulating body composition on outcomes. Although notoriously limited due to the complexity of modulating dietary intake, such studies should prospectively evaluate optimum dietary composition, the effect of BCAA supplementation, enteral tube feeding, vitamin D repletion, and the impact of exercise and physiotherapy.
In terms of current practical recommendations, clinicians should identify HCC patients at risk of sarcopenia at an early stage in their pathway and during follow-up appointments. Full anthropometric measures should be taken at regular intervals, including subjective global assessment and grip strength. BMI should not be used as a marker of malnutrition in such patients.12,13,36 Where available, the analysis of psoas muscle cross-sectional area at the initial staging CT scan would more accurately identify those with sarcopenia. Alternatively, the bioelectrical impedance measure phase angle has been shown to correlate with survival in HCC and can be measured at the bedside.36 Patients with underlying liver cirrhosis should receive adequate calorific intake as dictated by well-recognised international guidelines (for example, the European Society for Parenteral and Enteral Nutrition [ESPEN] guidance37). Patients with cirrhosis should also be considered for BCAA supplementation and the importance of a late evening snack should be re-enforced. Nutritional support in the form of oral nutritional supplements or nasogastric feeding should be administered if the patient is unable to meet their nutritional requirements. Patients being considered for hepatectomy should receive intensive preoperative dietary input and have a low threshold for preoperative enteral feeding. Finally, it goes without saying that all patients should receive regular dietary follow-up and have their nutritional statuses re-evaluated when a change in medical or surgical therapy is being considered.
CONCLUSION
To conclude, the available literature supports the adverse impact of sarcopenia and visceral fat deposition on overall survival, response to different treatment modalities, and tumour recurrence in HCC. There is a lack of studies investigating the optimum nutritional support for this condition and it is imperative that this field grows to complement future advances in medical therapy.








