Abstract
Necrolytic acral erythema (NAE) is a relatively newly described dermatologic disease that is often associated with hepatitis C virus (HCV). Oral zinc therapy is a successful treatment; however, therapy is often delayed due to misdiagnosis. There are limited reports of NAE in the literature. This paper presents a case of NAE in a 68-year-old male with untreated HCV, whose NAE was diagnosed and treated as recurrent cellulitis for 12 years. He had low serum zinc and elevated serum glucagon levels. Elevated glucagon is not often reported in NAE, but the patient’s CT abdomen was negative, ruling out glucagonoma and necrolytic migratory erythema. He improved with oral zinc replacement and was referred to the hepatology department for HCV treatment. This paper additionally presents a review of the literature for NAE cases.
INTRODUCTION
Necrolytic acral erythema (NAE) was first described in 1996 by el Darouti and Abu el Ela in Egypt.1 It is most often associated with hepatitis C virus (HCV) infection and is similar in morphology to other necrolytic erythemas.2 Classically, NAE presents as well-demarcated, dusky erythematous plaques over the dorsum of the feet and toes, with thickening of the plaques over time;3 however, NAE rarely develops in other areas such as the hands and elbows as well.2 While there have been more reports of NAE since its original designation, there is still a paucity of data regarding this entity. A case of NAE associated with elevated glucagon levels in a 68-year-old male with untreated HCV is presented here. His NAE was diagnosed and treated as recurrent cellulitis for 12 years.
CASE REPORT
The patient was a 68-year-old white, non-Hispanic male with a past medical history of untreated HCV, ex-intravenous (IV) drug abuse, chronic venous insufficiency, hypertension, tinea pedis, and recurrent left foot cellulitis, who was admitted to the hospital for progressive worsening of a painful left foot plaque. He reported that the lesion had started 3–4 weeks prior, but that he had experienced intermittent episodes of the same rash for the past 12 years, which had resolved with oral antibiotics, topical ketoconazole, and topical clotrimazole–betamethasone. The current plaque began localised to the left third digit and was associated with a burning pain. Prior to his current admission, he had been seen by multiple providers and had been unsuccessfully treated with oral cephalexin and topical mupirocin, doxycycline, and topical clotrimazole, and finally, IV vancomycin. Due to progression of his disease to his remaining left digits and proximal left foot, and new-onset redness and pain in his right foot, he was evaluated by podiatry who recommended he come to the emergency department for evaluation. He denied recent trauma or burns to his feet. His review of systems was not significant.
His past medical history was significant for untreated-HCV, ex-IV drug abuse, chronic venous insufficiency, hypertension, history of tinea pedis, and recurrent left foot cellulitis. He was diagnosed with HCV approximately 40 years prior and had never received treatment due to transportation and insurance barriers. His only medication was lisinopril.
On initial physical examination (Figure 1 and Figure 2) by the internal medicine team he had a well-demarcated, erythematous, ulcerated plaque extending to the dorsal surface with increased oedema on his left foot. There were macerations, fissuring, and sloughing of the epidermis at the plantar surfaces near the digits on both feet. Thickened nail plates with yellow discolouration and subungual debris were seen on multiple toenails, bilaterally. His leukocyte count and creatinine were elevated, and his serum albumin was low. His HCV antibody screen was >11 index (normal: ≤0.79) and his HCV RNA was 13,800,000 international units/mL (normal: <15 international units/mL). The patient was started on IV vancomycin and piperacillin/tazobactam, as well as oral terbinafine and topical clotrimazole–betamethasone for presumed left foot infection and right hallux infection. An X-ray was ordered, demonstrating some concern for osteomyelitis; a follow-up MRI was negative.
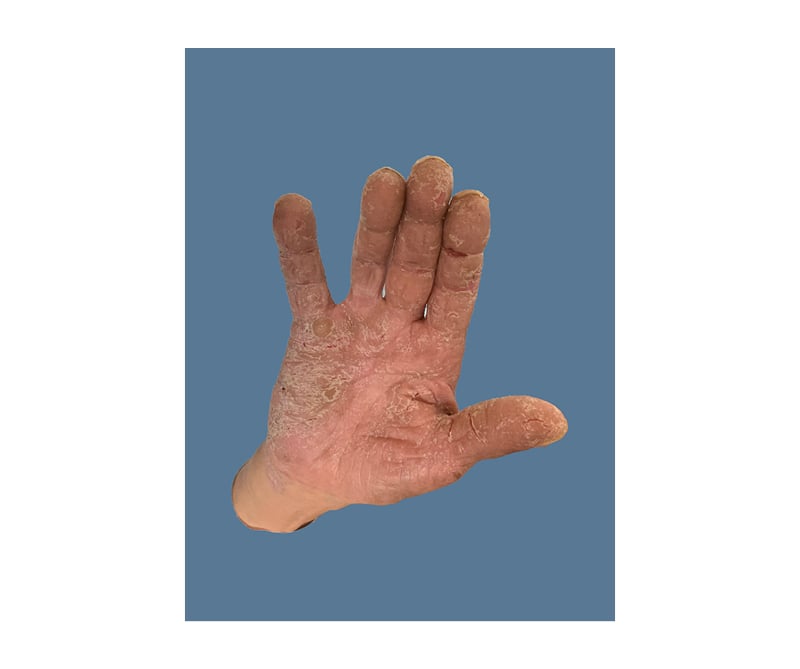
Figure 1: Necrolytic acral erythema affecting the right hand.
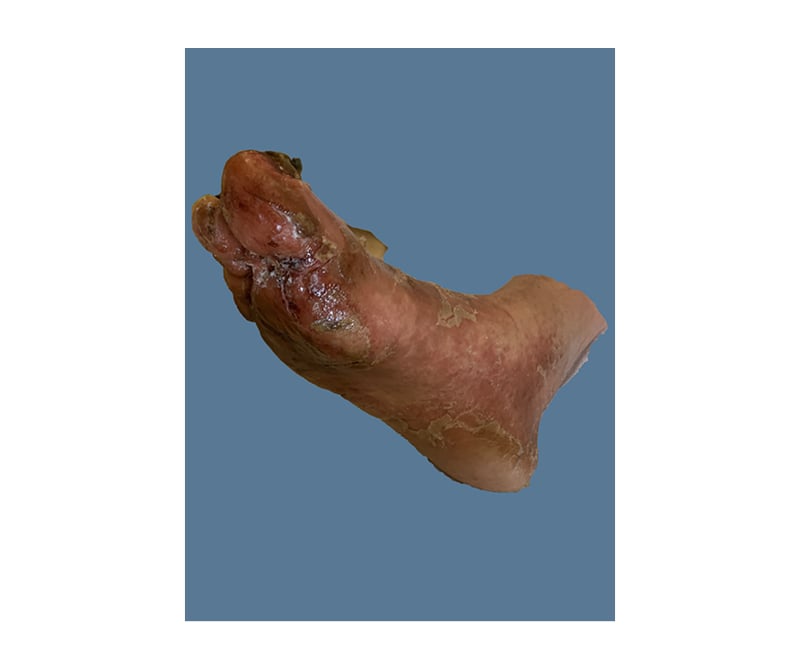
Figure 2: Necrolytic acral erythema affecting the right foot.
Due to failure to respond to antimicrobial therapy, dermatology was consulted on hospital Day 5. His physical examination at that time demonstrated dark erythema with superficial desquamation of bilateral dorsal and plantar feet and toes. Multiple toenails were thickened, with yellow discolouration and subungual debris. In addition, his bilateral hands, elbows, and heels had keratotic erythematous plaques with additional fissures and desquamation of his hands. He had fingernail clubbing. The remainder of his skin was clear. In addition to NAE, the differential diagnosis included lichen planus, psoriasis, atopic dermatitis, necrolytic migratory erythema (NME), niacin deficiency, acrodermatitis enteropathica, eczematous dermatoses, and tinea pedis.
The diagnosis of NAE was suspected based on clinical presentation of the hyperkeratotic plaques affecting his acral surfaces, as well as with his history of HCV.2 This diagnosis also remained the most likely when working through the differential diagnoses. While lichen planus does have a similar distribution, it has a characteristic violaceous colour and polygonal shape not seen in the patient.4 Psoriasis was not strongly considered as the patient’s rash did not have the typical silvery scale of psoriasis, but rather a very superficial desquamation.4 NME affects the perioral and peri-genital regions as well as the extremities (discussed below).5
Acrodermatitis enteropathica has a periorificial and peri-acral distribution and also often presents as a triad with diarrhoea and alopecia.4 Niacin deficiency can result in pellagra, which is characterised by the triad of dermatitis (typically affecting the neck or chest and hands), diarrhoea, and dementia.4 Simple eczema should also be included, but the rash was not blistering or oedematous.4 Tinea pedis was also considered; however, tinea does tend to be more inflammatory, with intense pruritus, brighter erythema, and finer scale (rather than the broad sheets of scale the patient had on his feet).4 He also had repeatedly failed treatment for tinea. While NAE was suspected, a biopsy was performed for more definitive diagnosis and laboratory tests were ordered for further evaluation.
The pathology report showed psoriasiform dermatitis with slight pallor in the superficial epidermis with foci of parakeratosis and serum, which was compatible with NAE given the corresponding clinical features. His glucagon level was elevated at 96 pg/mL (normal: 8–57 pg/mL) and his zinc level was low at 41 mcg/dL (normal: 60–130 mcg/dL). His cryoglobulin screen was negative and his rheumatoid factor, C3, and C4 levels were within normal limits. The clotrimazole–betamethasone, antibiotics, and terbinafine were discontinued and he was started on a zinc supplement and topical clobetasol. The patient improved to the point of being able to ambulate and there was no longer visible skin sloughing. He was discharged and scheduled for hepatology follow-up and a CT abdomen to definitively rule out glucagonoma due to his elevated glucagon level. The CT abdomen was negative for pancreatic lesions, which again verified the diagnosis of NAE as opposed to NME; the CT was also negative for hepatic cirrhosis and hepatocellular carcinoma. The patient is currently being seen by the hepatology service, with plans to start treatment for his HCV.
DISCUSSION
NAE is a relatively newly described pathology and is often associated with HCV.1 While it was once believed to be pathognomonic for HCV infection, it has since been reported in seronegative patients as well.3,6 While the exact incidence and prevalence of NAE is unknown, the majority of cases have been reported from Egypt, which has the highest prevalence of HCV in the world.7 Since its initial description in Egyptian patients, it has been reported in other countries, including the USA,3 India,6 Pakistan,8 and Canada, among others.9
On 13th January 2021, a literature search of the PubMed database was conducted using the search term ‘Necrolytic Acral Erythema’; all cases written in English were included (Table 1).1,3,6,9-47 There were 42 reports found, with a total of 63 cases of NAE described. Of these cases, 16 patients were black, two were white, three were Asian, and 42 did not explicitly list the patient’s race or ethnicity. The presented patient is white, non-Hispanic, which contributes to the epidemiology of NAE as it has mainly been described in the black population, although there is a paucity of data on race in the reports. Fifteen of the 16 cases reported in the black population were reports from the USA; this is likely because in the USA, HCV is more prevalent in African Americans than any other racial group.48 For the patients with an HCV diagnosis, it is unclear how long they had had the infection. At 68 years old, the patient is the same age as one patient and older than all but two of the reported cases; he had untreated HCV for approximately 40 years. While the exact pathogenesis of NAE is not yet known, it is hypothesised to be multifactorial, with contributions from zinc deficiency, hypo/hyperglucagonaemia, hepatic dysfunction, and hypoalbuminaemia, among others.2 The patient’s extensive history of untreated HCV, paired with his recurrent NAE, may suggest an association between infection duration and NAE. More studies need to be completed to evaluate for risk factors in NAE aetiopathogenesis.
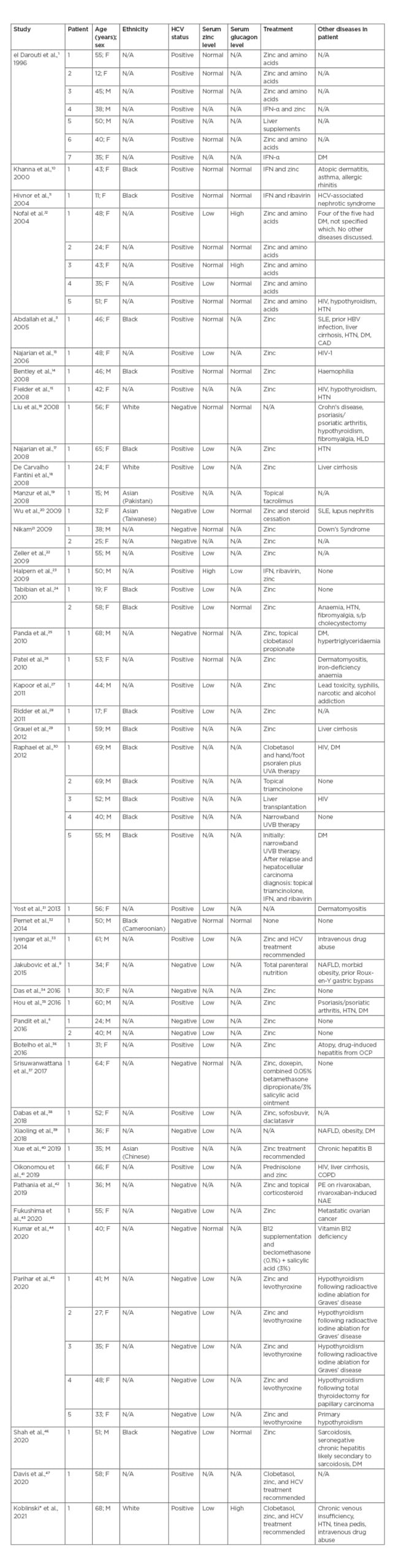
Table 1: PubMed-indexed cases of necrolytic acral erythema.
As aforementioned, NAE can be associated with an array of laboratory abnormalities. The patient had hypoalbuminaemia, zinc deficiency, and hyperglucagonaemia. Interestingly, NAE has been demonstrated to be responsive to zinc therapy in patients with both normal zinc levels10,11 and zinc deficiency.13,28 The patient was found to be zinc deficient and improved with zinc supplementation, contributing to the proposed link between zinc deficiency and NAE. Zinc levels should be checked in all patients with suspected NAE.
The link between NAE and disruption in glucagon levels is often discussed, especially due to the similar entity NME. NME is the dermatosis associated with glucagonoma syndrome and presents as painful erythematous plaques that coalesce into bullous lesions; it affects the perioral and peri-genital regions as well as the extremities.5 While NAE and NME are similar in morphology, they have different body distributions.2 In addition, the two have similar findings under histopathological evaluation (as well as overlapping features with pellagra and acrodermatitis enteropathica), with neither having pathognomonic features.2,49,50 Both conditions can reveal parakeratosis, acanthosis, psoriasiform hyperplasia, and superficial epidermal necrosis depending on stage of disease and biopsy site.2,49,50 These similarities demonstrate how clinical correlation as well as laboratory tests and imaging are necessary for a more definitive diagnosis. For further distinction between the two, patients with NME typically have glucagon levels >500 pg/mL,5 while patients with NAE can have normal,10 low,16 or, less often reported, high levels of glucagon.12 The patient was found to have an elevated glucagon of 96 pg/mL (normal: 8–57 pg/mL).
While this raised suspicion for NME, the patient’s dermatologic findings were in a distribution consistent with NAE and he had a negative CT abdomen. Of the PubMed-indexed cases, only two cases reported by Nofal et al.12 had high glucagon levels, so this is an infrequently reported (or potentially under-reported) finding. For those two cases, the actual values of their elevated glucagon were not listed, but both did have a negative CT scan of the pancreas as well as HCV.12 There is debate about whether NAE and NME are distinct entities or if NAE is truly a variant of NME, especially due to the similar histopathologic findings,12 and the mildly elevated glucagon level in the patient may suggest the latter. Whether the two pathologies are different or on a spectrum, glucagon levels should be checked when either is suspected. An elevated glucagon level warrants imaging to rule out glucagonoma. It has been hypothesised that in NAE, elevated serum glucagon induces inflammation through high levels of arachidonic acid and its metabolism.28
The patient’s diagnosis of NAE had been missed for 12 years resulting in significant morbidity. It was affecting his quality of life and he had been exposed to unnecessary and costly treatment throughout this time. As the incidence and prevalence are unknown, it is unclear how many other patients with NAE may be misdiagnosed as well. It is important for providers to be able to recognise this entity, especially as it is treatable with zinc replacement and, if indicated, HCV therapy.2 This will help to avoid unnecessary morbidity for patients. Patients with HCV especially should be counselled about the cutaneous manifestations of HCV, including NAE, and told to report any concerns to their provider. As more cases are reported, the understanding of the epidemiology of NAE will increase and potentially lead to better recognition and care.
CONCLUSION
NAE is important to have on the differential diagnoses for patients with well-demarcated, erythematous acral plaques, especially in a patient with HCV. Misdiagnosis of NAE can lead to ineffective and unnecessary treatment and increased patient morbidity. If NAE is suspected, laboratory tests for HCV status as well as serum zinc and glucagon levels are warranted, and this case highlights elevated glucagon levels can be found in patients with NAE. Oral zinc is often a successful treatment and, if indicated, referrals to hepatology for HCV therapy are necessary.








