Meeting Summary
Despite significant therapeutic advances in the treatment of patients with non-Hodgkin lymphoma (NHL), a significant proportion experience relapse or progression following standard immunochemotherapy (ICT). The introduction of novel targeted immunotherapy agents has potentially ushered in a new era in the management of NHL. Emerging approaches to treatment, including chemo-free regimens, targeted therapies, and immunotherapy for follicular lymphoma (FL), mantle cell lymphoma (MCL), and diffuse large B-cell lymphomas (DLBCL), have become increasingly important. Furthermore, genomic tools and biomarkers support subtyping of lymphomas and contribute greatly to identifying patients likely to respond to therapy and predict treatment outcome, thus offering a subset-specific precision medicine approach to managing NHL to both prevent and treat relapse. The latest development in the management of NHL is the use of checkpoint inhibitors to prevent cell–cell communication and tumour growth. Despite limited evidence to date, checkpoint inhibitors in combination with existing ICT may fundamentally shift the NHL treatment algorithm towards personalised immunotherapy.
Follicular Lymphoma: Strategies in the Era of New Targeted Therapies
Professor Dolores Caballero
Despite advances in the understanding of the pathophysiology of FL and the number of available therapeutic options, this disease remains incurable. FL is the second most common B-cell lymphoma and >80% of patients are diagnosed with advanced Stage III–IV disease. Although the average survival is 15 years, it has been shown that over time survival rates decrease from 82% at 5 years to 71% at 10 years.1 Furthermore, projected survival rates and treatment outcomes dramatically worsen in patients who transform to DLBCL.2
Available first-line treatment options for patients with FL include observation and ICT, depending on tumour burden at diagnosis. However, in patients with asymptomatic advanced FL, rituximab resulted in significantly greater time to new treatment initiation and progression-free survival (PFS) compared with observation (p<0.0001) but had no significant impact on overall survival (OS) (p=0.40).3 In patients with a high tumour burden, 2 years of maintenance following ICT was associated with a better PFS (59.2% versus 42.7%; p<0.0001), but again without an advantage on OS when compared with no maintenance.4 In patients with a high tumour burden, the combination of rituximab and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) may be the preferred first-line treatment option. R-CHOP was superior to rituximab, and CVP (cyclophosphamide, vincristine, prednisolone) in time to treatment failure and PFS over 3 years had a better risk:benefit ratio compared with rituximab in combination with fludarabine and mitoxantrone,5 and was noninferior to bendamustine plus rituximab with regard to CR rate in the BRIGHT trial.
Overall, rituximab combined with ICT has led to improved PFS when used as first-line, relapse, or maintenance treatment. However, the cytotoxic burden of combined ICT can have a negative impact on patients’ quality of life. Furthermore, FL is a heterogeneous condition that requires tailored treatment approaches. Therefore, emerging targeted chemotherapy-free immunological strategies are promising and have the potential to personalise FL treatment and reduce cytotoxicity. Novel therapies targeting crucial pathways (e.g. BTK inhibitors, PI3K inhibitors, and BCL2 antagonists), cellular therapies (e.g. CAR-T cells), and new monoclonal antibodies could change the treatment landscape.
Obinutuzumab
In patients who are refractory to rituximab, the anti-CD20 antibody obinutuzumab, in combination with bendamustine, significantly improved PFS (hazard ratio: 0.55; p<0.0001) with similar OS (79% versus 77%; p=0.74), compared with bendamustine monotherapy.7 Similarly, in the GALLIUM study, first-line combination therapy with obinutuzumab-chemotherapy demonstrated significantly higher 3-year PFS than R-chemotherapy (80% versus 73%; p=0.0012) in patients with indolent NHL.8
Idelalisib
Idelalisib is a PI3K inhibitor that was associated with greater PFS than the most recent prior therapy used in patients who were refractory to rituximab and an alkylating agent. Improved PFS and prognosis were reported in patients with a complete response (CR) to idelalisib. Therefore, idelalisib was recommended as suitable in high-risk, heavily pre-treated patients with relapsed/refractory FL. The most frequently reported Grade ≥3 treatment-emergent adverse events (AEs) were diarrhoea (14%), pneumonia (7%), and pyrexia (4%).9,10
Ibrutinib
Some ICT-resistant patients with FL receiving ibrutinib, a BTK inhibitor, reported clinical benefits with low level AEs. Durable responses were achieved by 21% of patients with a median time to response of 5.7 months and median PFS of 4.6 months.11 Combination therapy with rituximab or rituximab and bendamustine resulted in an 82% overall response (OR) rate in treatment-naïve patients with 27% achieving CR, or 90% OR with 50% of
patients experiencing CR, respectively.12,13
Lenalidomide
The immunomodulatory agent lenalidomide combined with rituximab (R2) increases the number of CD4 and CD8 T cells, natural killer (NK), and natural killer T (NKT) cells in peripheral blood after six treatment cycles,14 aiding the recruitment of other immune cells to induce tumour cell apoptosis (Figure 1).15
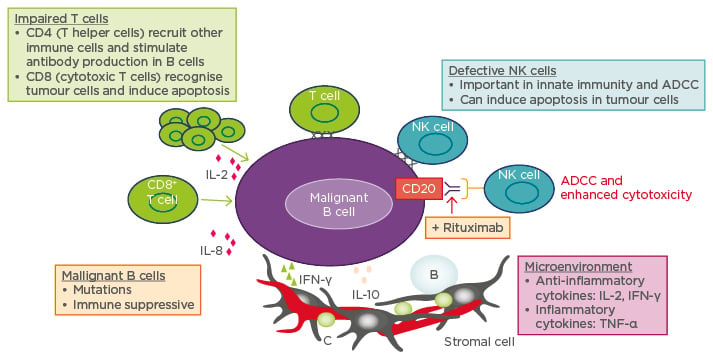
Figure 1: Mechanism of lenalidomide in lymphoma: Direct killing and immune modulation.
ADCC: antibody-dependent cellular cytotoxicity; IFN-γ: interferon-γ; IL: interleukin; NK: natural killer;
TNF-α: tumour-necrosis factor alpha.
R2 was more efficacious than lenalidomide monotherapy in patients with recurrent FL, with R2 resulting in a slower rate of disease progression (p=0.002), a higher OR (76% versus 53%), and a higher CR rate (76% versus 20%).16 R2 also resulted in higher PFS and OS in untreated patients with indolent FL.14
In the Phase II SAKK 35/10 study, R2 as first-line treatment demonstrated higher OR than rituximab monotherapy (82% versus 61% by Week 23) in high-risk FL patients, described as clinically significant progression over ≥6 months, bulky disease ≥6 cm in diameter, and clinically significant progressive anaemia/thrombocytopenia.17 In these patients, R2 was also associated with higher median PFS (p=0.03) and a significantly improved complete response rate at 30 months (42% versus 19%; p=0.001), but 3-year OS rates were similar between the groups (93% versus 92%). However, R2 was associated with higher risk of neutropenia (23% versus 7%) and 11/77 patients discontinued treatment due to toxicity. In this specific study, lenalidomide toxicity may be related to increased risk with continuous dosing.17
Combining more than two agents in FL (e.g. rituximab, lenalidomide, and ibrutinib/idelalisib) was associated with higher risk of AEs, including rash, neutropenia, and hepatoxicity, regardless of patient treatment or relapse history.18-20
Randomised trials assessing lenalidomide in combination with rituximab are ongoing.21 Although traditional ICT combinations have been effective prior to relapse, novel non-cytotoxic targeted therapies have shown more efficacy than rituximab monotherapy, with a more tolerable safety profile. Next generation treatments aim to combine biologic and targeted agents to create personalised combinations, but mechanistic and biomarker studies are required.
Shaping Treatment Approaches in Diffuse Large B-Cell Lymphoma Using Molecular Subtyping
Professor Umberto Vitolo
DLBCL is the most common subtype of NHL, accounting for about 40% of all lymphomas. DLBCL is heterogeneous and complex, and often classified on the basis of morphology, immunophenotype, genetic alterations, and clinical behaviour. Although R-CHOP is the foundation for treatment currently in the first-line setting, treatment outcomes vary and 40–50% of patients relapse.22 The potential for relapse depends on clinical subtypes, which are poorly defined. There are currently several biomarkers used to classify subtypes of DLBCL and predict treatment outcome in DLBCL patients. Some of the most important include cell of origin (COO), MYC/BCL2 co-expression, and MYC/BCL2 translocations.
Cell of Origin
The World Health Organization (WHO) 2016 report23 requires that diagnosis of DLBCL includes identification of the COO subtype, and while using immunohistochemical (IHC) algorithms is acceptable, gene-expression profiling methods are preferred. Although IHC-based algorithms for distinguishing between GCB and non-GCB types are easy to use and relatively inexpensive, they have low reliability and variable-reported accuracy, lack of standardisation, and high inter-observer variability. Gene expression-based assays range from genome-wide assays to targeted approaches, differing according to platform, number of genes recorded, base model, RNA input, and misclassification rates. The NanoString Lymph2Cx 20-gene assay is one example of a simple and fast technique for distinguishing between germinal centre B-cell-like (GCB), activated B-cell-like (ABC), and unclassified types of DLBCL, with a 2% chance of misassignment.24 However, while gene-expression profiling is more reliable and reproducible when compared with IHC-based algorithms, it is costly, not widely available, and requires more time to operate. Thus, incorporation and standardisation of COO analysis in routine clinical practice remains a challenge.
COO subtype has been shown to be predictive of treatment response. For example, ABC DLBCL has been reported to have lower probability of PFS and OS than GCB DLBCL in response to R-CHOP (p<0.001) in both retrospective studies,25 and also in the recent prospective GOYA study, which randomised DLBCL patients to receive R-CHOP or obinutuzumab combined with CHOP (G-CHOP).26 Identification of COO is also important in the salvage setting, as shown by the BIO-CORAL study, where rituximab in combination with dexamethasone, high-dose cytarabine, and cisplatin as salvage therapy (R-DHAP) improved PFS compared to rituximab in combination with ifosfamide, carboplatin, and etoposide (R-ICE) in the GCB subgroup (52% versus 31%, respectively; p=0.01),27 but without a noticeable difference between the two groups in the ABC subgroup (p=0.82).26,27
However, there are drug candidates being tested that could improve the prognosis in ABC DLBCL patients. For example, the ABC subtype combination therapy of ibrutinib and R-CHOP showed promising treatment outcomes in ABC types with untreated CD20+ B-cell NHL: 94% OR with 72% of patients achieving a CR and the most common Grade 3 AEs were thrombocytopenia (21%), anaemia (18%), and febrile neutropenia (18%).28 Ongoing Phase III trials are currently investigating whether these treatment benefits can be seen in patients with newly diagnosed non-GCB-type DLBCL receiving R-CHOP in combination with ibrutinib and R-CHOP alone.
Lenalidomide, another therapy with potential preferential activity in non-GCB types, in combination with R-CHOP, was associated with greater event-free survival predominantly in patients with ABC-type DLBCL compared with patients who were given R-CHOP alone (69% versus 48%), while no significant difference was observed between treatments in GCB-type patients (67% versus 71%).29,30 Furthermore, R2-CHOP (lenalidomide, rituximab, CHOP) demonstrated an OR of 92–98%, with 80–86% of patients experiencing CR.30,31 The most common AEs associated with lenalidomide were Grade 4 neutropenia (55–75%), leukopenia (37–48%) and thrombocytopenia (17–18%), but overall toxicity was not much different from that expected with standard R-CHOP.30,31 The ongoing randomised ROBUST study comparing R-CHOP ± lenalidomide in ABC DLBCL patients, screened with NanoString technology is designed to confirm whether R2-CHOP benefits patients with the ABC DLBCL subtype.
MYC/BCL2 Co-expression and MYC/BCL2 Double Translocations
The WHO 2016 classification23 introduced a new category of DLBCL called ‘high grade B-cell lymphoma with MYC and BCL2 and/or BCL6 translocations’, which includes all double-hit or triple-hit lymphomas and recognised dual gene translocations of MYC and BLC2 as a novel prognostic marker of double-hit lymphoma.
Studies have shown that patients with double-hit lymphoma have worse treatment outcomes than those with only one of the two genes or none, regardless of induction treatment regimen.32 In double-hit lymphoma patients, the PFS and OS rates at 2 years were 40% and 49%, respectively. In contrast, in patients overexpressing MYC/BCL2, 3-year PFS was 63% versus 89% in those not overexpressing either gene.33 Three-year PFS was 68% in patients overexpressing only BCL2, which is similar to the rates seen in dual expressor patients. On the other hand, 3-year PFS was 74% in patients overexpressing only MYC, which suggests that poor prognosis of double expressor lymphoma is mainly driven by the over-expression of BCL2.33 In addition, most patients with dual expressor lymphoma tend to belong in the ABC category; however, COO did not provide statistically significant risk stratification within patients with MYC/BCL2 co-expression.25
Gene expression and genome sequencing analyses have the ability to not only distinguish DLBCL subtypes and the molecular basis of chemotherapy resistance, but also possibly provide insights into novel targets for therapeutic interventions incorporating biological agents to the R-CHOP backbone. This may allow for subtype/subset-specific precision medicine and personalised combinations to both prevent and treat relapsed/ refractory DLBCL in the future. However, additional complexities in molecular subtyping, including double-hit, triple-hit, double expressers, epigenetics, and other oncogenes, in specific cases further complicate clinical practice.
Mantle Cell Lymphoma:Evolving Treatment Strategies
Professor Martin Dreyling
MCL is a rare form of NHL that generally affects older individuals. It is a heterogeneous disease ranging from indolent (15% of cases); to the more prevalent classical MCL (80%), characterised by initial high response rates, but rapid relapses; to the rarer transformed blastoid lymphoma (5%), which is the most aggressive type and is associated with the poorest prognosis of all the lymphomas.
Current standard of care (SOC) guidelines for patients older than 65 years recommend either R-CHOP, bendamustine and rituximab, or bortezomib combined with CHOP. The optimal treatment is to achieve remission with ICT as first-line therapy, followed by rituximab maintenance with or without autologous stem cell transplant to eliminate minimal residual disease.
SOC guidelines for younger patients (≤65 years) include dose intensification combined with cytarabine. Substituting three cycles of R-CHOP with three cycles of R-DHAP increased molecular remissions (70% versus 37% in bone marrow), which translated into improved time to treatment failure (p=0.038).34 High dose consolidation with autologous stem cell transplant is generally recommended for younger patients.35 In addition, rituximab maintenance is associated with significantly higher rates of event-free survival and OS compared with observation.36
The introduction of maintenance therapy after first-line therapy is altering the potential treatment outcome for patients, extending the time to relapse. However, despite remission being frequently achieved with first-line treatment, MCL becomes more aggressive once relapse occurs. Therefore, targeted approaches should be considered at first relapse and recommended for higher relapse patients in both age groups (Figure 2).
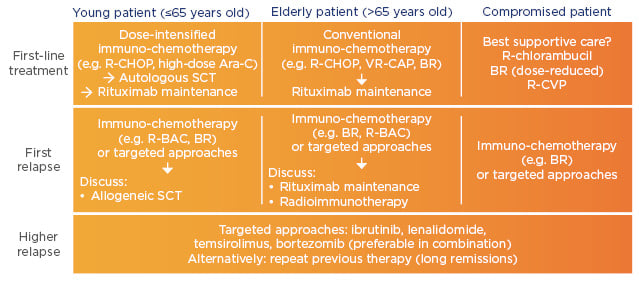
Figure 2: Clinical recommendations for mantle cell lymphoma.37
Ara-C: cytarabine; BAC: bendamustine, cytarabine; BR: bendamustine, rituximab; CAP: cyclophosphamide, doxorubicin, prednisone; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; CVP: cyclophosphamide, vincristine, prednisone; R: rituximab; SCT: stem cell transplant; VR: bortezomib, rituximab.
Based on their ability to prolong remission duration, B cell tyrosine kinase receptor-targeting drugs (ibrutinib) and immunomodulatory drugs (lenalidomide) may be used as monotherapy in the relapsed/refractory setting, whereas, temsirolimus and bortezomib are recommended in combination with chemotherapy. Data from a head-to-head trial confirms that temsirolimus should preferably be administered as combination therapy.38 In patients with relapsed or refractory MCL, ibrutinib monotherapy increased the likelihood of achieving PFS by 57% relative to temsirolimus monotherapy, with two-fold higher median PFS rates (14.6 versus 6.2 months, respectively; p<0.0001).38 Similarly, in another study of relapsed/refractory MCL, lenalidomide monotherapy resulted in superior PFS relative to chemotherapy, with a median PFS of 8.7 versus 5.2 months (p=0.004).39
Despite recent evidence, non-chemotherapy approaches do not adequately overcome all risk factors associated with MCL, thus combination treatment rather than monotherapy may be the next logical step. Venetoclax achieves high response rates of 75% in patients with NHL, but the duration of remission is unknown.40 In the Phase II AIM study, ibrutinib and venetoclax have produced encouraging outcomes in patients with relapsed/ refractory MCL.41 The combination of ibrutinib and R2 results in 88% response rate including those patients previously treated with ibrutinib.42
The European MCL network is currently testing two of the most effective registered compounds in first-line. Young patients receive the current SOC (rituximab and cytarabine containing induction followed by autologous stem cell transplant and rituximab maintenance) +/- ibrutinib. In elderly patients, an alternating R-CHOP/high dose cytarabine induction is followed by rituximab maintenance +/- lenalidomide.
Emerging Role of Checkpoint Inhibitors in Non-Hodgkin Lymphoma
Professor Roch Houot
Checkpoint inhibitors are antibodies that bind to specific antigens or receptors on the surface of T cells or cancer cells to prevent their communication and subsequent activation of downstream effectors in the immune system. For example, anti-CTLA-4 antibodies bind to the CTLA-4 receptor on the surface of the T cell, which prevents the B7 antigen on the surface of dendritic cells from binding to the CTLA-4 receptor during the priming phase. During the effector phase, T cells communicate with cancer cells via PD-1 receptors on the T cell and corresponding PD-L1 receptors on the cancer cell. Antibodies targeted at both receptors block receptor interaction and prevent cell–cell communication (Figure 3).43
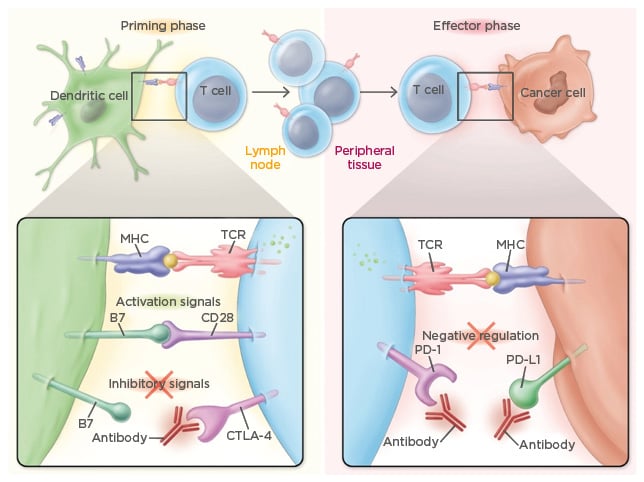
Figure 3: Mechanism of action of checkpoint inhibitors.
CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; MHC: major histocompatibility complex; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; TCR: T cell receptor.
Despite significant therapeutic advances in the treatment of patients with NHL, a significant proportion of patients experience relapse or progression following standard ICT. The introduction of novel immunotherapy agents, specifically targeting cell–cell communication, has ushered in a new era in the management of NHL. Biological and early clinical data suggest that some subgroups of NHL patients may be particularly sensitive to checkpoint inhibitors. In addition, combination therapies that include checkpoint inhibitors may further increase the number of NHL patients who may benefit from these immunotherapies.
Ipilimumab, an anti-CTLA-4 antibody, was shown to be well tolerated in a Phase I trial with a clinical response in 11% of patients and a biological response (defined as T cell proliferation to recall antigens) in 31% of patients with relapsed/refractory B-cell NHL.44 Nivolumab and pembrolizumab, anti-PD-1/PD-L1 antibodies, demonstrated 68% and 69% OR, respectively, in patients with relapsed/refractory classical HL.45,46
It is hypothesised that the reason anti-PD-1 antibodies trigger such high OR in patients with HL is that HL tumour cells continuously express PD-L1 receptors, which is caused by a genetic alteration in the 9p24.1 chromosome. Ninety-seven percent of these alterations contain the genes PD-L1 and PD-L2, which are directly expressed at the surface of tumour cells, and JAK2, which indirectly induces expression of PD-L1 and PD-L2. However, 40% of HLs are associated with the Epstein-Barr virus (EBV), which can induce PD-L1 expression via the LMP1 protein.47-50
Patients with B-cell NHL have lower response rates than those with HL (~28% for nivolumab, similar to the rates of those with other solid tumours).51 However, some NHL subtypes may be particularly sensitive to PD-1 blockade treatment. Solid tumour cells in general are more likely to respond to antiPD-1 antibodies if they are immunogenic and if they express PD-L1 ligands. The presence of PD-L1 expressing tumour cells was associated with higher OR rates when patients were given nivolumab (36% versus 0% of patients not expressing PD-L1 in their solid tumour cells).52 Solid tumours with neoantigens or high mutational load respond better to checkpoint inhibitors since they are the most immunogenic.53-55 Mutational load in lymphoma B cells ranges from occasional to regular,56 with mutational loads in FL and DLBCL being comparable to those of lung and bladder cancer;57 thus, it is presumed that lymphoma B-cells with the most regular mutational load will be most responsive to anti-PD-1 antibodies.
The 9p24.1 chromosome alteration was also found in subtypes of NHL, such as primary mediastinal large B-cell lymphoma (55%), primary testicular lymphoma (54%), and EBV-negative primary central nervous system lymphoma (52%).58 Unsurprisingly, DLBCL subtypes with positive EBV expression were also associated with high PD-L1 expression since EBV induces PD-L1 expression. For example, 94% EBV-positive DLBCL cells expressed PD-L1, whereas DLBCL not otherwise specified showed only 11% PD-L1 expression.59
The Phase II KEYNOTE-170 trial showed 41% OR in primary mediastinal large B-cell lymphoma patients who were given pembrolizumab along with ≤50% tumour reduction in 73% of patients.60 Similar outcomes were observed in patients with NK/T cell lymphoma. Response rates directly correlated with PD-L1 expression and a positive response was observed in 100% of patients with 5/7 patients experiencing CR.61 Another trial showed that all five patients with relapsed/refractory primary central nervous system lymphoma or primary testicular lymphoma responded to nivolumab, with four patients experiencing CR.62
The most common, all-grade, immune-related AEs related to anti-PD-1 antibodies were fatigue (25%), infusion-related reaction (20%), and rash (16%). The most common immune-related AEs Grade ≥3 were increased lipase levels (6%) and neutropenia (5%).45,63 Severe toxicities were observed when anti-PD-1 antibodies were given before or after allogeneic haemostatic stem cell transplant, such as severe acute graft-versus-host disease, and it is therefore not recommended to administer anti-PD-1 treatment until several months after allogenic transplant.64-66
Although the evidence supporting their role in management of NHL is still limited, the use of checkpoint inhibitors (alone or in combination) in patients with NHL is likely to represent an important therapeutic option in some subgroups of patients allowing for tumour regression and prolonged remission, notably in chemorefractory patients.







