Meeting Summary
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death in the world, accounting for approximately 1.4 million new cases and almost 700,000 deaths in 2012.1 The objective of the symposium was to provide an overview of the current treatment landscape in terms of later-line therapy in metastatic CRC (mCRC) and to discuss the evidence for the various options available, including rechallenge and therapies such as trifluridine (FTD)/tipiracil (TPI) (Lonsurf®; also known as TAS-102) and regorafenib (Stivarga®). The symposium started by examining the clinical value of third-line treatment in patients with mCRC and providing an insight into the mechanism of action of FTD/TPI, and a comparison with that of 5-fluorouracil (5-FU). The safety and efficacy of FTD/ TPI was then discussed together with the practical management of patients on treatment. The speakers tackled the issue of rechallenge and reintroduction as an option in the third-line, reviewing the pros and cons, and the available studies providing information on the safety and efficacy of the different options in later lines, concluding that there is a lack of robust evidence for rechallenge as a clinical decision. This was followed by a review of the compelling evidence for the use of treatments such as FTD/TPI and regorafenib in the third-line, with documented evidence for efficacy.
Welcome and Introduction
Professor Dirk Arnold
According to the 2016 European Society for Medical Oncology (ESMO) guidelines for the treatment of mCRC, first and second-line treatments comprise mainly a combination of chemotherapy (e.g. fluoropyrimidines with oxaliplatin and/or irinotecan) and monoclonal antibodies ([mAb]; e.g. bevacizumab, cetuximab, panitumumab, ramucirumab) or aflibercept.2 In recent years, the proportion of patients who are candidates for treatment beyond the second-line, i.e. fit enough to receive treatment, according to the current ESMO guidelines, has increased to 50–60%.2 This is likely due to the improvements in earlier treatment lines, as more and more patients remain in good performance status with controlled disease conditions. Treatment options beyond second-line include single agents such as FTD/TPI and regorafenib, as well as mAb and combination therapy for patients who have not previously received an anti-epidermal growth factor receptor (EGFR) antibody. The challenge is how to determine the appropriate treatment for use given the options available.
CLINICAL VALUE OF THIRD-LINE TREATMENTS IN PATIENTS WITH METASTATIC COLORECTAL CANCER
Understanding the Added Value of Trifluridine/Tipiracil
Professor Andres Cervantes
FTD/TPI is indicated for the treatment of adult patients with mCRC who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapies, anti-vascular endothelial growth factor agents, and anti-EGFR agents.3 It is currently approved in the USA, the European Union (EU), Japan, and Argentina.3-6 The use of FTD/TPI in the third-line setting in mCRC is recommended in national and international guidelines such as ESMO, National Comprehensive Cancer Network (NCCN), National Institute for Health and Care Excellence (NICE), and the Japanese Society for Cancer of the Colon and Rectum (JSCCR).2,5,7,8
Mechanism of Action of Trifluridine/Tipiracil
FTD/TPI is a novel oral antitumour nucleoside; it is made up of FTD, a thymidine-based nucleoside analogue, and TPI, a thymidine phosphorylase inhibitor, at a molar ratio of 1:0.5. FTD exerts its cytotoxic effects primarily by incorporation of its active metabolite, FTD triphosphate (trifluoromethyl deoxyuridine 5’-triphosphate [F3dTTP]), into DNA.9-11 Under normal circumstances, systemic FTD is subject to hepatic, thymidine phosphorylase-mediated degradation; co-administration of TPI inhibits thymidine phosphorylase, thus increasing the bioavailability of FTD (37-fold increase in exposure) for conversion to the active metabolite within cells.9,12
Entry of FTD into the cancer cell (via active transportation or nucleoside transporters) is followed by phosphorylation by thymidine kinase to ultimately produce F3dTTP (Figure 1). F3dTTP is readily incorporated into the DNA of tumour cells (in the place of thymidine bases), thus interfering with DNA function and inhibiting cell proliferation and tumour growth.9 The mechanism of action of FTD is in contrast with that of 5-FU, a uracil-based nucleic acid analogue, which exerts its antitumour activity by inhibition of DNA synthesis via depletion of deoxythymidine triphosphate (dTTP) (due to inhibition of thymidylate synthase) (Figure 1).9
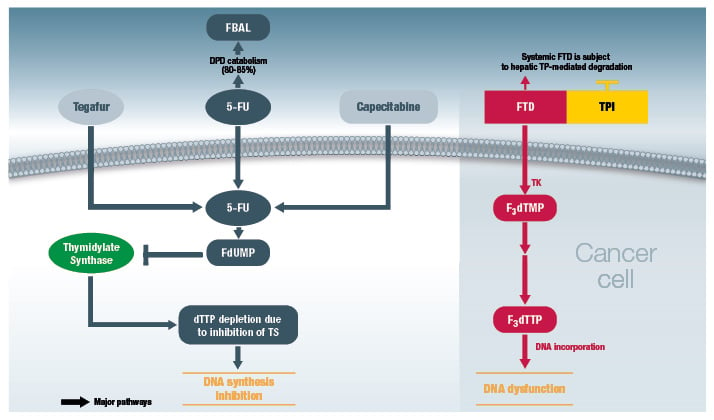
Figure 1: Comparison of mechanisms of action of trifluridine/tipiracil (right-hand panel) versus 5-FU (left-hand panel).9,13
5-FU: 5-fluorouracil; DPD: dihydropyrimidine dehydrogenase; dTTP: deoxythymidine triphosphate; FBAL:
alpha-fluoro-beta-alanine; F3dTMP: trifluoromethyl deoxyuridine 5’-monophosphate; F3dTTP: trifluoromethyl deoxyuridine 5’-triphosphate; FdUMP: fluorodeoxyuridine monophosphate; FTD: trifluorothymidine (trifluridine); TK: thymidine kinase; TP: thymidine phosphorylase; TPI: tipiracil hydrochloride; TS: thymidylate synthase.
© Les Laboratoires Servier, 2017 (published with permission).
Interestingly, in preclinical studies, FTD/TPI demonstrated antitumour activity in 5-FU-sensitive as well as 5-FU-resistant cells.14 This observation was carried through in the RECOURSE study, which demonstrated that FTD/TPI was also clinically active in patients who are refractory to 5-FU.15 In summary, DNA dysfunction by FTD/TPI is distinct from the mechanism of action of 5-FU, resulting in antitumour activity of FTD/TPI in both 5-FU-sensitive and resistant tumours.
Clinical Efficacy and Safety of Trifluridine/Tipiracil
Professor Marc Peeters
The median overall survival (OS) for patients with mCRC is currently >30 months, as a result of the availability of new drugs as well as the multidisciplinary approach to the continuum of care.2 Moving from first-line to later treatment lines, it is important to achieve a balance, not just between the efficacy and safety of treatments, but also the quality of life provided by these treatments. In the third-line setting, two drugs (FTD/TPI and regorafenib) have demonstrated efficacy versus best supportive care (BSC) in heavily pretreated patients with mCRC in randomised Phase III trials. However, no head-to-head data for these two options are available.
Data from three clinical trials are available for FTD/ TPI: a Phase II trial (J003, N=172),16 the Phase III RECOURSE trial (N=800),15 and the Phase III TERRA trial, which was an East Asian specific study (N=406).17 The participants in these trials were patients with refractory disease having previously received ≥2 regimens prior to study entry and with Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 in the majority of patients. The patients were randomised in a 2:1 ratio of FTD/TPI with BSC versus placebo with BSC. Patients randomised to FTD/TPI received a dose of 35 mg/m2 twice daily on Day 1 up to Day 5, and then from Day 8 until Day 12, every 4 weeks.
Overall, the three studies showed the following with regard to efficacy:15-17
- Significant and clinically meaningful improvements in median OS were observed with FTD/TPI versus placebo, with hazard ratios (HR) ranging from 0.56–0.79.15-17 In the RECOURSE study, the OS benefits of FTD/TPI were observed not only in the overall population but also across different patient subgroups.15
- FTD/TPI offered a significant and clinically meaningful improvement in progression-free survival (PFS) compared with placebo, with HR ranging from 0.41–0.48;15-17 PFS benefits were also consistent across all prespecified subgroups in the J003 and RECOURSE trials.15,16
- FTD/TPI offered consistent improvements in disease control rate compared with placebo (about 44% versus about 15%).15-17
- FTD/TPI effectively prolonged time to deterioration of ECOG performance status from 0–1 to ≥2 (5.7 versus 4.0 months for placebo) in the RECOURSE study.15
– 84% of patients remained at ECOG performance status 0–1 at treatment discontinuation.18
With regard to safety, the RECOURSE study showed the following:15
- FTD/TPI had a well-tolerated safety profile with a low rate of dose reductions, discontinuations, and severe adverse events (AEs).
- Overall, only 4% of patients on FTD/TPI withdrew due to AEs (versus 2% for placebo), while 14% required a dose reduction.
- Treatment with FTD/TPI was well tolerated with minimal non-haematological AEs (with the most common being nausea/vomiting, decreased appetite, fatigue, and diarrhoea).
- The main AEs associated with FTD/TPI were haematological in nature (neutropenia, leukopenia, and anaemia), which were generally manageable; guidelines are available for the management of these AEs.3
These safety results were consistent with what has been observed in the other two trials.16,17
Appropriate dose management is important for optimal clinical efficacy. With regard to haematological events, complete blood cell counts must be obtained prior to initiation of each cycle. In the event of neutropenia and thrombocytopenia, treatment with FTD/TPI should be interrupted and resumed only on recovery of neutrophil counts to ≥1.5 × 109/L and platelets to ≥75 × 109/L, respectively. Details for the management of neutropenia are provided in Figure 2. Similar guidance is recommended with regard to the management of Grade 2/3 and Grade 4 thrombocytopenia.
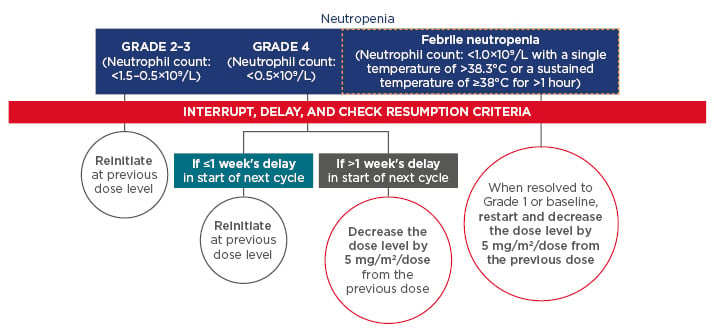
Figure 2: Recommendations for dose delay and dose reduction for patients with neutropenia.3
Resumption criteria (CTCAE, Grade 1 or better): neutrophils, ≥1.5 × 109/L; platelets, ≥75 × 109/L. Resumption criteria applied at the start of the next cycle for all patients regardless of whether or not the interruption criteria were met.
CTCAE: Common Terminology Criteria for Adverse Events.
© Les Laboratoires Servier, 2017 (published with permission).
With regard to non-haematological AEs, the recommendation for most Grade 3 or 4 events would be to interrupt treatment and re-initiate with a dose 5 mg/m2 lower than the previous dose when the AE is resolved (to baseline levels). The exceptions to this recommendation are Grade 3/4 nausea and/or vomiting events that can be controlled by antiemetic therapy, and diarrhoea that can be managed with antidiarrhoeal medicinal products. In the majority of cases, it may not be necessary to decrease the dose of FTD/TPI.
These recommendations are in line with the observation that only 4% discontinuation due to AEs were reported in the RECOURSE trial, which demonstrates the effectiveness of management of AEs with dose delay and reduction strategies. In summary, FTD/TPI is effective as a treatment option for mCRC beyond second-line, with a favourable safety profile.
TREATMENT CHOICE AT THIRD-LINE:TO RECHALLENGE OR NOT TO RECHALLENGE?
Rechallenge in the Continuum of Care
Professor Dirk Arnold
According to the 2016 ESMO guidelines, there is an option of the usage of either new single agents, or a rechallenge or reintroduction of treatment of mCRC beyond the second-line.2,19 Rechallenge refers to the reinitiation of a therapy (after an intervening period) to which the tumour had proved to be resistant in earlier-line treatments.19 By contrast, reintroduction refers to the reinitiation of treatment without evidence of disease progression in the interval; this may be according to a set duration (e.g. adjuvant treatment) or following a planned treatment break (e.g. for the reduction or management of AEs).
The rationale for rechallenge lies in the observation that cancer is a clonal disease and that tumour progression is generally not homogeneous. It is feasible that some clones that were sensitive to first-line treatment, but had escaped elimination, would re-emerge as clones that would be the source of tumours that progress following second-line treatment. It stands to reason that the first-line treatment may therefore have a clinical effect on the tumour in the third-line setting.
In the OPTIMOX studies, previously untreated patients were randomly assigned to either a combination of leucovorin (LV) and fluorouracil (FU) with oxaliplatin (FOLFOX4) administered every 2 weeks until progression, or a simplified LV and FU regimen with high-dose oxaliplatin (FOLFOX7) for six cycles, maintenance without oxaliplatin for 12 cycles, and reintroduction of FOLFOX7. The pooled analysis of OPTIMOX-1 and OPTIMOX-2 suggests that reintroduction after a ≥6-month oxaliplatin-free interval improves PFS, OS, and response rates in treatment-naïve patients with mCRC.20 Similar results were also observed in more complex treatment regimens such as LV and FU and oxaliplatin and irinotecan (FOLFOXIRI) treatment, with response rates of 38% following rechallenge in patients who have progressed following maintenance treatment.21
A similar observation was also noted for cetuximab rechallenge in patients with irinotecan-refractory mCRC who had a clinical benefit after initial cetuximab plus irinotecan therapy but experienced disease progression following treatment with both cetuximab and a second-line of chemotherapy. Rechallenge resulted in an overall response rate of ≤54%.22 Molecular analyses of circulating tumour cells and circulating tumour DNA have demonstrated the reappearance of anti-EGFR-sensitive tumour cell clones after ceasing targeted therapy,23 suggesting that rechallenge with anti-EGFR agents may be a viable option for these patients.
Nonetheless, examination of the available literature on rechallenge with anti-EGFR in mCRC indicates limited evidence for this option, as the majority of studies are retrospective in nature with poor levels of evidence (Level IV).22,24-26 A similar situation is observed with the concept of retreatment with oxaliplatin, with poor evidence levels for the available studies. In summary, reintroduction of treatment can produce a high overall response rate and may represent a viable option in certain circumstances.21 While the mechanisms of action supporting rechallenge are not completely understood and the levels of evidence are poor, oxaliplatin rechallenge appears to be a viable option in mCRC (with oxaliplatin-free interval ≥6 months).20
Third-line Treatment in the Continuum of Care
Professor Julien Taieb
While the treatment goals for mCRC vary according to the different lines of systemic treatment, the treatment goal for patients receiving third-line treatment and beyond is the maintenance of good quality of life and performance status (given their short life expectancy). Indeed, treatment-related factors (toxicity) take on a more important role (compared with tumour/disease-related characteristics and patient-related factors) as a patient progresses through the continuum of care.
It appears that the evidence of rechallenge with chemotherapy and targeted agents is conflicting; very little data are available on irinotecan and there are no data on bevacizumb beyond the second-line. Evidence for oxaliplatin comprises mostly of reports on a first-line stop-and-go (intermittent) treatment strategy (FOLFIRI) rather than third-line rechallenge. A study comparing patients receiving interval chemotherapy between the first FOLFOX and second FOLFOX therapy or having a chemotherapy holiday demonstrated PFS and OS of 27 and 58 weeks, respectively, after a chemotherapy holiday, compared with 11 and 36 weeks, respectively, after interval chemotherapy.27 These results demonstrate that clinical outcomes are worse with rechallenge compared with reintroduction of oxaliplatin.
Similarly, the evidence for rechallenge with anti-EGFR mAbs is conflicting. On the one hand, results of a Phase II, prospective study of KRAS wild type patients (N=39) with mCRC show that rechallenge with cetuximab plus irinotecan may achieve clinical benefit (objective response rate: 53.8% [complete response: 5.1%; partial response: 48.7%]) and delay disease progression in patients who had previously experienced clinical benefit.19,22 On the other hand, panitumumab monotherapy has shown minimal benefit in patients with KRAS wild type mCRC (N=20) that has progressed on prior cetuximab.28 In addition, it has been shown that response to rechallenge with anti-EGFR is influenced by the response to the first use as well as the interval between the first and second anti-EGFR treatment; prior responders with longer interval length were more likely to respond to anti-EGFR.29 Finally, it is well known that RAS mutations are present in >50% of all mCRC, which would preclude the use of anti-EGFRs for rechallenge.29
In this situation, options that are available for patients ineligible for anti-EGFR rechallenge are the single agents regorafenib and FTD/TPI. The CORRECT study demonstrated the clinical benefits of regorafenib on OS (HR: 0.77; 95% confidence interval [CI]: 0.64–0.94; p=0.0052) and PFS (HR: 0.49; 95% CI: 0.42–0.58; p<0.0001) compared with placebo in third-line treatment of patients with mCRC.30 The RECOURSE study demonstrated significant efficacy benefits with FTD/TPI on OS (HR: 0.68; 95% CI: 0.58–0.81; p<0.001, with a 2-month improvement in median OS), and PFS (HR: 0.48; 95% CI: 0.41–0.57; p<0.001) versus placebo.15 It should be noted that treatment with FTD/TPI is not considered as a 5-FU rechallenge, as the mechanisms of action of the individual treatments are different (Figure 1).9 This has been confirmed in the RECOURSE study, which demonstrated the clinical effects of FTD/TPI in patients who had progressed on 5-FU.15 In particular, treatment with FTD/TPI prolonged the PS of patients compared with placebo, with almost 9 out of 10 patients retaining a PS of 0–1 at the end of the study.15
Currently, the ESMO 2016 consensus guidelines recommend that treatment be given at third-line followed by a rechallenge (but not conversely).2 These recommendations for treatment with regorafenib or FTD/TPI in the third-line setting (ahead of rechallenge) are supported by the strong evidence for the clinical efficacy of regorafenib18,30,31 and FTD/TPI15-17 in prospective clinical trials in the third-line setting. In summary, evidence-based treatments that are recommended in national and international guidelines should be the preferred third-line treatment options.2,7
Summary and Conclusion
Professor Andres Cervantes
The main points of the symposium are summarised in Figure 3.
Figure 3: Summary of symposium.
ESMO: European Society for Medical Oncology; OS: overall survival; PS: performance status; RCT:
randomised clinical trial.







