Speakers: Ajay Kakkar,1 Jeffrey I. Weitz,2 Harry R. Büller,3 Jean M. Connors4
1. Thrombosis Research Institute, London, UK
2. McMaster University, Hamilton, Canada
3. Academic Medical Center, Amsterdam, The Netherlands
4. Harvard Medical School, Boston, Massachusetts, USA
Disclosure: Kakkar has received research support from Bayer and Sanofi; and personal fees from Bayer, Sanofi, and Anthos Therapeutics. Weitz has received research support or acted as principal investigator from Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, and Canadian Fund for Innovation; has served as a consultant for Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, Portola Pharmaceuticals, Ionis Pharmaceuticals, Janssen Pharmaceutica, Merck & Co., Novartis, Anthos Therapeutics, and Tetherex Pharmaceuticals; and is on the scientific advisory board for Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, Portola Pharmaceuticals, Ionis Pharmaceuticals, Janssen Parmaceutica, and Laboratoires Servier. Büller has received research support or acted as principal investigator from Sanofi-Aventis, Bayer HealthCare, Bristol Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline (GSK), Pfizer, Roche, Ionis, Boehringer Ingelheim, Eli Lilly, and Novartis; and has served as a consultant and on the scientific advisory board for Sanofi-Aventis, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, GSK, Pfizer, Roche, Ionis Pharmaceuticals, Boehringer Ingelheim, Eli Lilly and Company, Novartis, and Anthos Therapeutics. Connors has served on scientific advisory boards and as a consultant for Abbott, Anthos Therapeutics, Alnylam® Pharmaceuticals, Bristol Myers Squibb, Portola Pharmaceuticals, and Takeda; and has received research funding to the institution from CSL Behring.
Acknowledgements: This report was written by Jenny Lloyd, Compass Medical Communications, Ltd., UK.
Disclaimer: The views and opinions expressed are those of the speakers and not necessarily those of Anthos Therapeutics.
Support:The publication of this article was funded by Anthos Therapeutics.
Citation: EMJ. 2021;6[4]:12-20.
Summary
Ajay Kakkar opened this virtual meeting by discussing the history of the treatment and prevention of thrombosis. Early anticoagulants (unfractionated heparin and warfarin) reduced thrombosis risk but increased bleeding risk. Direct oral anticoagulants (DOACs) were more effective than warfarin and associated with a lower, but still present, bleeding risk. Jeffrey I. Weitz discussed the traditional view of the coagulation cascade, in which pathological thrombosis (i.e., harmful clots that occlude blood vessels) and physiological haemostasis (i.e., bleeding prevention) appear to be inextricably linked. He then introduced a more recent model with two distinct pathways, in which pathological thrombosis and physiological haemostasis could be uncoupled if certain factors are targeted, reducing bleeding risk. Factor XI is a potential target, with strategies for its inhibition including antisense oligonucleotides, monoclonal antibodies (e.g., abelacimab, osocimab), aptamers, and small molecules (e.g., asundexian, milvexian). Harry R. Büller presented key trial data in patients undergoing total knee arthroplasty. Compared with enoxaparin, abelacimab significantly reduced the risk of venous thromboembolism (VTE) after a single post-operative 150 mg dose (4% versus 22%; p<0.001). FXI-ASO also reduced VTE compared with enoxaparin (4% versus 30%; p<0.001), but after nine 300 mg subcutaneous doses. Lastly, a single 1.8 mg/kg pre-operative dose of intravenous osocimab reduced VTE versus enoxaparin, but to a lesser extent (11% versus 26%; p<0.001). Jean M. Connors concluded that factor XI is a promising new target for the inhibition of pathological thrombosis, with minimal impairment of physiological haemostasis. The potential applications of factor XI inhibitors were then debated in a panel discussion.The Prevention and Treatment of Thrombosis: Six Decades of Progress
Ajay Kakkar
There has been substantial progress in the field of venous and arterial thrombosis in the last 60 years. Back in 1960, Barritt and Jordan1 published a study showing that the administration of an anticoagulant to patients with pulmonary embolism could significantly reduce death from this condition, from 5/19 untreated patients to 0/54 treated patients (p=0.0007). In the 1970s, the focus switched from the treatment of VTE to its prevention. In 1975, an international multicentre trial of 4,121 patients undergoing elective major surgical procedures showed that the administration of low-dose unfractionated heparin significantly reduced the number of patients with fatal pulmonary embolism compared with control (2 versus 16; p<0.005).2
A meta-analysis published in 2007 included six randomised trials, published during the 1990s, of patients with atrial fibrillation (AF) who had been randomised to warfarin or control/placebo.3 Among a total of 2,900 patients, warfarin reduced the risk of stroke compared with control/placebo by 64% (95% confidence interval [CI]: 49–74).3 A later meta-analysis included four Phase III randomised trials of patients with AF, published during 2009–2013, which compared direct oral anticoagulants, also referred to as non-vitamin K antagonist oral anticoagulants, with warfarin.4 Among a total of 71,683 patients, DOACs significantly reduced the risk of stroke or systemic embolic events by 19% (95% CI: 9–27) compared with warfarin.4 Together, these studies established the principle of intervention to prevent thromboembolic stroke with an anticoagulant in patients with AF.
However, anticoagulants can increase the risk of bleeding, particularly in certain patient groups. The GARFIELD-VTE registry included 10,684 patients with VTE, who were followed for up to 3 years.5 Patients with active cancer were at increased risk of major bleeding (hazard ratio [HR]: 3.8; 95% CI: 2.9–5.0) compared with patients without cancer. Patients with active cancer also had an increased bleeding risk compared with patients with a history of cancer (HR: 2.9; 95% CI: 1.7–5.0).5
In the GARFIELD-AF registry, 52,032 patients with newly diagnosed AF were given a vitamin K antagonist (VKA) (±an antiplatelet agent), a DOAC (±an antiplatelet agent), an antiplatelet agent alone, or no antithrombotic treatment.6 Various factors were significantly associated with increased bleeding risk, including a history of bleeding (HR: 2.38; 95% CI: 1.72–3.30), moderate or severe chronic kidney disease (HR: 1.72; 95% CI: 1.41–2.10), older age (HR: 1.23; 95% CI: 1.18–1.29), and anticoagulant used.6 In terms of the anticoagulant, the bleeding risk was higher with a VKA than with a DOAC, and lowest with an antiplatelet agent alone.6 The bleeding risk increased with the addition of an antiplatelet agent to a VKA or a DOAC.6 Further, patients with major bleeding had a higher risk of all-cause mortality than those without major bleeding (adjusted HR [aHR]: 8.24; 95% CI: 6.76–10.04).6 The presence of less severe bleeding also had an impact on all-cause mortality, including clinically relevant non-major bleeding (aHR: 2.59; 95% CI: 1.80–3.73) and minor bleeding (aHR: 1.53; 95% CI: 1.07–2.19).6
In GARFIELD-AF, both the proportion of patients receiving an anticoagulant and the choice of anticoagulant changed over time.7 During 2010–2011, approximately 57% of patients were given an anticoagulant (either a VKA or DOAC),7 but by 2015–2016 this had increased to around 71% (unpublished data). Among the patients who received an anticoagulant, this was mainly a VKA (approximately 93%) during 2010–2011, with approximately 7% receiving a DOAC.7 By 2015–2016, patients were more likely to receive a DOAC (approximately 61%), with approximately 39% receiving a VKA (unpublished data). Most importantly, even in 2015–2016, approximately 25% of patients with AF, who are at increased risk of stroke, were not receiving any anticoagulant (unpublished data).
Even with the availability of DOACs, which have a lower bleeding risk than VKAs, the risk of major bleeding remains a deterrent to optimal anticoagulation, and prescription remains suboptimal. For example, in the NCDR PINNACLE registry, approximately 50% of outpatients with AF who were considered to be at high stroke risk (CHA2DS2-VASc: ≥5) received no anticoagulant.8 Similarly, 40% of patients admitted to hospital with pre-existing AF (and CHA2DS2-VASc: ≥2) were receiving no anticoagulation at the time of admission.9 Findings among other patient types at risk of VTE are similar, with 42% of surgical inpatients and 60% of non-surgical inpatients receiving no anticoagulation.10
In conclusion, despite substantial progress in the treatment and prevention of venous and arterial thrombosis over the last six decades, and the validation of the efficacy and safety of DOACs in clinical practice, there remains an unmet clinical need due to the risk and fear of bleeding. Therefore, there is a need for new anticoagulants with a lower bleeding risk.
Uncoupling Haemostasis from Thrombosis: The Potential of Factor XI Inhibition
Jeffrey I. Weitz
In traditional coagulation cascade models, the contact activation (or intrinsic) pathway (activated by factors inside the vascular system) and the tissue factor (or extrinsic) pathway (activated by external trauma) converge. This implies that pathological thrombosis and physiological haemostasis, both of which depend on clot formation, are inextricably linked through the common pathway. However, if just the contact pathway could be inhibited, this could be used to attenuate thrombosis with minimal disruption of haemostasis, thus reducing bleeding risk.
Various new initiators of the intrinsic pathway have been described. These are naturally occurring polyanions and include neutrophil extracellular traps extruded from activated leukocytes, DNA and RNA released from activated or damaged cells, and inorganic polyphosphates released from activated platelets. These anionic polyanions activate factor XII and induce thrombosis through the contact pathway.
Various lines of evidence support factor XI as an antithrombotic target. Firstly, individuals with factor XI deficiency (activity: ≤50%) have a reduced risk of VTE (aHR: 0.26; 95% CI: 0.08–0.84).11 While such individuals can have some increased bleeding risk, this is rarely spontaneous or severe.12,13 Secondly, individuals with elevated factor XI levels (above the 90th percentile) have a 2.2-fold higher adjusted risk (95% CI: 1.5–3.2) of deep venous thrombosis (DVT).13 Thirdly, various animal studies support factor XI as an antithrombotic target with low bleeding risk.14
Strategies to target factor XI include: antisense oligonucleotides (ASO), which reduce hepatic synthesis of factor XI; aptamers, which bind factor XI and block activity; antibodies, which bind factor XI and block activation or activity; and small molecules, which bind reversibly to the active site of factor XIa and block activity.15
As mentioned above, the traditional view of the coagulation cascade suggests that the generation of the fibrin in an occlusive thrombus inside a vessel, or the fibrin in a haemostatic plug that seals leaks in damaged vessels both occur via a connected coagulation pathway. This led to the belief that it is not possible to have effective anticoagulation without an appreciable bleeding risk.
However, newer models of the coagulation cascade have delineated two distinct pathways, with only one main section in common: the downstream common pathway (Figure 1).16 In this new model, haemostasis (i.e., the formation of a plug to seal leaks in damaged blood vessels) is mainly an extravascular process, triggered by high concentrations of tissue factor in the haemostatic envelope that surrounds blood vessels. Therefore, with high tissue factor concentrations, fibrin production occurs via the extrinsic pathway (left pathway in Figure 1). Pathological thrombosis (i.e., a clot inside a blood vessel) is triggered by much lower concentrations of tissue factor that are exposed when atherosclerotic plaques are disrupted or are presented on the activated endothelium that tethers tissue factor-expressing monocytes or microvesicles on the surface. With low concentrations of tissue factor, clotting initially occurs through the extrinsic pathway. However, the intrinsic pathway becomes very important for thrombus growth due to the back activation of factor XI by thrombin; and the activation of factor IX intensifies factor X activation and resultant fibrin formation (right path in Figure 1).16
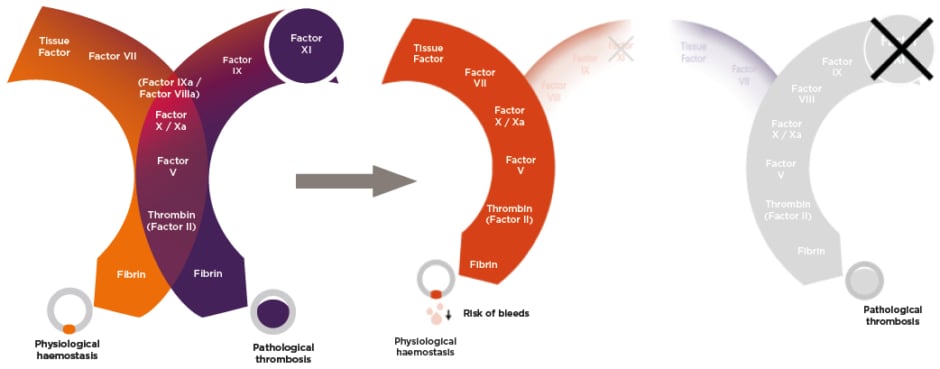
Figure 1: Newer model of the coagulation cascade.16
DOACs and warfarin target factor X/Xa and prothrombin/thrombin (factor II/IIa), both of which are in the common pathway (centre of Figure 1). Warfarin additionally targets a number of other factors. Hence, DOACs and warfarin impact haemostasis as well as thrombosis. However, targeting factor XI, which is intimately involved in thrombosis but is generally non-essential for haemostasis, provides an opportunity to pharmacologically ‘uncouple’ the two coagulation pathways. This could enable the effective suppression of the pathological thrombosis pathway, while leaving the physiological haemostasis pathway largely unaffected, thus reducing bleeding risk.
Factor XI-directed strategies are being tested in various Phase II studies, as recently reviewed by Fredenburgh and Weitz.17 Agents include monoclonal antibodies (e.g., abelacimab, osocimab), ASOs, and small molecules (e.g., asundexian, milvexian) (Table 1).17 Abelacimab binds to factor XI and blocks its activation by factor XIIa and thrombin, whereas osocimab only inhibits factor XIa.17 Both monoclonal antibodies can be given subcutaneously or intravenously, as single doses or once per month. They have a rapid onset and slow offset of action, and neither requires renal clearance nor poses a risk of drug–drug interactions. ASOs decrease factor XI synthesis, can be given weekly to monthly, and have a slow onset of action.17 The small molecules inhibit factor XIa and are given orally, but require daily administration due to their rapid offset of action. They also undergo some renal clearance and have the potential for drug–drug interations.17
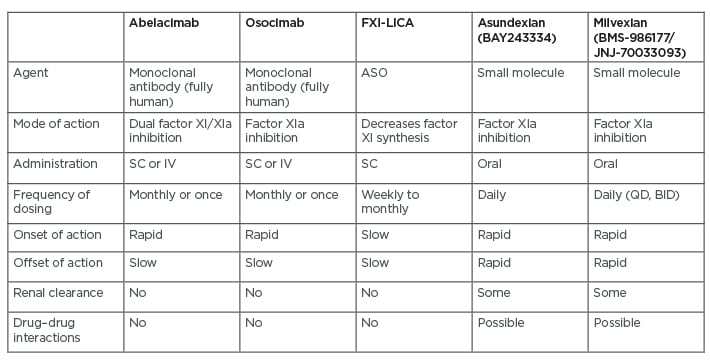
Table 1: Drugs that target factor XI.
ASO: antisense oligonucleotide; BID: twice daily; FXI-LICA: factor XI ligand-conjugated antisense; IV: intravenous; SC: subcutaneous, QD: once daily. Adapted from Fredenburgh and Weitz.17
A potential new indication for factor XI inhibitors is the prevention of major adverse cardiovascular events in patients with end-stage renal disease (with or without AF), which could be highly beneficial as such patients cannot take DOACs as they are cleared via the kidneys, thus increasing the risk of accumulation and consequent bleeding. Factor XI inhibitors may also provide a safer platform for antiplatelet therapy in patients with acute coronary syndromes, secondary stroke prevention, the prevention or treatment of cancer associated VTE, and prevention of thrombosis on devices such as central venous catheters, mechanical heart valves, and left ventricular assist devices.
In conclusion, factor XI is emerging as a promising target for new anticoagulants. Several strategies to inhibit factor XI are under investigation, and ongoing trials will determine their benefit–risk profile and whether they can uncouple thrombosis and haemostasis.
Abelacimab: A Dual Factor XI/XIa Inhibitor
Harry R. Büller
Abelacimab is a unique, fully human monoclonal antibody that inhibits both the inactive (zymogen) and active forms of factor XI.18 It binds to the catalytic domain of both factor XI and factor XIa with high affinity and selectivity.18 It has two mutations in the fragment crystallisable region, which reduce the potential for fragment crystallisable-γ receptor binding and complement activation.
In the ANT-005 TKA study,19 412 patients who were undergoing total knee arthroplasty were randomly assigned 1:1:1:1 to a single intravenous infusion of post-operative abelacimab (30, 75, or 150 mg), or to once daily subcutaneous enoxaparin (40 mg). The primary efficacy outcome was objectively documented symptomatic VTE or asymptomatic DVT on mandatory venography on Day 10 (±2).19 The principal safety outcome was a composite of major and clinically relevant non-major bleeding up to Day 30 after surgery.19 At baseline on Day 1 prior to surgery, the median factor XI activity was approximately 120% in each of the three abelacimab dose groups. On Day 3, factor XI activity was <10% in all patients in all three dose groups. By Day 10, factor XI activity remained ≤10% in all patients in the two higher dose groups, while it had increased to a median of around 55% in the lowest dose group. By Day 30, median activity in the 150, 75, and 30 mg dose groups was approximately 5%, 75%, and 100%, respectively.19 Rates of VTE were lower with all doses of abelacimab (4%, 5%, and 13%, with decreasing doses) than with enoxaparin (22%), reaching significance (p<0.001) in the two highest dose groups (Figure 2).19
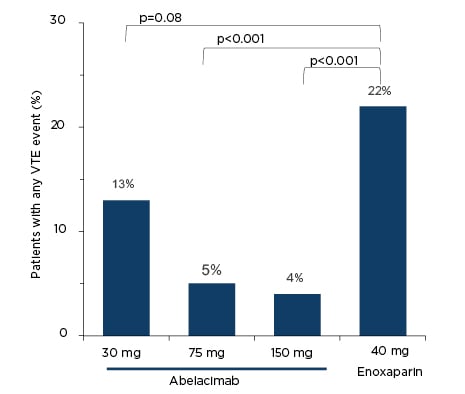
Figure 2: Rates of venous thromboembolism in the ANT-005 TKA study.19
VTE: venous thromboembolism.
Symptomatic VTE only occurred in one patient (in the enoxaparin group); the other 43 patients who met the primary endpoint had asymptomatic DVTs. Most of the DVTs were distal, with only three proximal DVTs (one in the abelacimab 30 mg group and two in the enoxaparin group).19 Only four patients had a major or clinically relevant non-major bleed, two in each of the two lower abelacimab dose groups. Only one of these events was classified as major bleeding. It occurred in a patient in the 75 mg abelacimab group, who had two bleeding events: a clinically relevant non-major bleed and a joint infection and haemarthrosis that was classified as a major bleed.19
Other published clinical trials of factor XI inhibition in patients undergoing total knee arthroplasty include the FXI-ASO TKA study20 and the FOXTROT osocimab trial.21 In the FXI-ASO TKA study,20 patients received nine subcutaneous doses of FXI-ASO (200 or 300 mg) during 36 days before to 3 days after surgery or enoxaparin 40 mg daily from the day before or the day of surgery for at least 8 days. VTE occurred in 4% of patients in the 300 mg group, 27% in the 200 mg group, and 30% in the enoxaparin group (p<0.001 for 300 mg versus enoxaparin).20 In the FOXTROT trial, patients received a single intravenous dose of osocimab (0.3, 0.6, 1.2, or 1.8 mg/kg) on the day after surgery, a single intravenous dose of osocimab (0.3 or 1.8 mg/kg) on the day before surgery, daily enoxaparin (40 mg), or twice daily apixaban (2.5 mg).21 The lowest rate of VTE occurred in the pre-operative osocimab 1.8 mg/kg group (11%), a rate significantly lower than that in the enoxaparin group (26%; p<0.001).21
Other ongoing or completed Phase II clinical trials of drugs targeting factor XI include those testing factor XI ligand-conjugated antisense (NCT04534114)22 or osocimab (NCT04523220)23 in patients with end-stage renal disease, milvexian in patients undergoing total knee arthroplasty (NCT03891524)24 or for secondary stroke prevention (NCT03766581),25 and asundexian in patients with AF (NCT04218266),26 non-cardioembolic stroke (NCT04304508),27 or acute myocardial infarction (NCT04304534).17,28
An ongoing Phase II study of abelacimab (ANT-006 AZALEA-TIMI 71) is comparing it with rivaroxaban in 1,200 patients with AF (NCT04755283).29 There are also two planned Phase III trials in patients with cancer-associated thrombosis, one comparing abelacimab with apixaban in approximately 1,600 patients and one comparing it with dalteparin in approximately 1,000 patients.
In conclusion, current data support the concept of factor XI inhibition for reducing the risk of thrombosis. Rates of VTE after knee surgery with factor XI inhibitors are impressively low, with no evidence for increased risk of bleeding in this setting. Further, post-operative factor XI inhibition is very effective. Current data indicate that abelacimab could be a promising new drug in this field, and future studies will hopefully provide further support.
Conclusion
Jean M. Connors
Anticoagulants can significantly reduce the risk of stroke in patients with AF and reduce the risk of thromboembolic events. They are excellent treatments for acute thrombosis but can increase the risk of bleeding, which is of particular importance in patients with cancer. The avoidance of anticoagulants due to this increased bleeding risk has not been resolved with the introduction of DOACs. Further, patients at perceived risk of bleeding often receive inappropriately reduced doses of DOACs, which can reduce efficacy without necessarily reducing bleeding risk.
Factor XI is a promising new target for the inhibition of pathological thrombosis with minimal impairment of physiological haemostasis. Phase II data with abelacimab demonstrate impressive efficacy in patients undergoing total knee replacement, with no safety signals. Overall, abelacimab is a promising new treatment in many clinical situations to inhibit thrombosis without impairing haemostasis.
Panel Discussion
Abelacimab could potentially be useful in a range of patient populations including those with cancer-associated thrombosis, AF, or VTE. Patients with increased bleeding risk often also have an increased risk of thrombosis, making the uncoupling of physiological haemostasis and pathological thrombosis particularly important. Factor XI inhibitors could also be a safer option for reducing major adverse cardiovascular events in patients with end-stage kidney disease, who are at increased risk of bleeding. They may also be useful for preventing recurrent stroke in patients with non-cardioembolic stroke, in patients with acute coronary syndromes, or to prevent clotting on central venous catheters, etc. Current data on drugs targeting factor XI support the uncoupling of the coagulation model, with these drugs able to achieve very low VTE rates without increasing the risk of bleeding.
A major benefit of abelacimab over ASOs is that it can be given as a single dose on the day of surgery, rather than having to start approximately 1 month before a planned procedure. The possibility of once monthly subcutaneous dosing for other indications could also improve patient adherence, increase treatment satisfaction, and reduce patient burden. Monoclonal antibodies also have no risk of drug–drug interactions or issues with hepatic metabolism or renal clearance, thus improving safety. They can be given intravenously, if a very rapid onset of action is required, or subcutaneously for longer-term use.
In the real world, it is important to balance efficacy and safety. Current data indicate that abelacimab has very good efficacy, coupled with a favourable safety profile, making it a very promising candidate. The dosing of conventional anticoagulants is limited by bleeding risk, but if factor XI inhibitors can uncouple physiological haemostasis and pathological thrombosis, it may be possible to get high efficacy without increasing bleeding risk.
Much of the residual thromboembolic burden of thromboembolic disease is because, in trying to achieve safety with current drugs, physicians tend to under-dose, putting patients at risk of thromboembolic events. There are also patient populations who currently receive no anticoagulation due to their elevated bleeding risk. The factor XI inhibitors may prove particularly beneficial in both these populations.
The GARFIELD-AF registry highlights the suboptimal uptake of anticoagulants among patients with AF.7 With a safer option available, both physicians and patients may be more likely to prescribe/accept treatment. Although aspirin is often perceived to have a lower bleeding risk, this is not actually true.
In conclusion, factor XI inhibition with abelacimab could potentially offer the holy grail of targeting and inhibiting pathological thrombosis and separating it from physiological haemostasis. These attributes could benefit many patients who require anticoagulation but are not receiving it due to bleeding concerns.







