Abstract
Hypomethylating agents (HMA) azacitidine and decitabine are standard of care for the treatment of myelodysplastic syndromes (MDS). Although HMA have revolutionised the treatment of MDS, only approximately half of patients respond to these agents with variable duration of effect, known as primary and secondary HMA failure, respectively. Therapeutic options following HMA failure remain limited; however, growing understanding of the pathogenesis underlying MDS has resulted in the development of multiple targeted therapies showing varying degrees of success in clinical trials. Drugs that target molecular alterations (such as abnormal histone regulation, IDH mutations, and spliceosome gene mutations), abnormal signalling pathways (such as the multikinase inhibitor rigosertib), cellular apoptosis (such as the Bcl2 inhibitor venetoclax), and immune checkpoint inhibition are under development. Agents recently approved for use in higher-risk acute myeloid leukaemia, such as FLT3-inhibitors and CPX-351, are also being studied in MDS. Several more agents, including two first-in-class agents, a novel immune regulator targeting CD47, and pevonedistat, a NEDD8-activating enzyme inhibitor, are under investigation. In the absence of established therapeutic approaches following HMA failure, decisions in therapy should be based on the type of HMA resistance as well as the patient’s clinical and molecular characteristics. As targeted therapies continue to be developed, a comprehensive re-evaluation of the patient including the mutational profile at the time of HMA failure may reveal new treatment options. Here, emerging therapeutic approaches to HMA failure in MDS are reviewed.
INTRODUCTION
Myelodysplastic syndromes (mds) are a heterogeneous group of disorders defined by ineffective haematopoiesis and clonal instability with risk of transformation to acute myeloid leukaemia (AML).1 Goals of therapy are to reduce the symptom burden from cytopenias and decrease the risk of progression of disease. Only three drugs have been approved by the U.S. Food and Drug Administration (FDA) for use in MDS: lenalidomide, an orally administered immunomodulatory drug; and two parenterally administered nucleoside analogue hypomethylating agents (HMA), azacitidine and decitabine. HMA have been the standard of care for patients with MDS for over a decade.2,3 Azacitidine, first approved in 2004, received expanded approval in 2008 for patients with higher-risk MDS based on the large, randomised Phase III AZA-001 trial which showed a median overall survival (OS) of 24.5 months compared to 15.0 months in patients receiving supportive care.2 Decitabine was approved in 2006 based on Phase III study results showing an overall response rate (ORR) of 17% compared to 0% of patients receiving supportive care.3 However, only azacitidine has been shown to prolong survival in MDS. While the European Medicines Agency (EMA) has approved HMA for International Prognostic Scoring System (IPSS; Table 1)4 intermediate and higher-risk MDS, some countries such as the USA also utilise these agents in lower-risk patients. A revised IPSS (IPSS-R; Table 2) was developed in 2012; however, established therapies were approved using the original IPSS system.5 Recent data suggests a benefit to early intervention in lower-risk patients.6 Although HMA prolong survival, the response is transient and as many as half of patients will not respond to these agents. Moreover, there are different types of HMA failure, including absence of response, or progression of disease or failure following an initial response, termed primary and secondary failure, respectively. Regardless of the nature of failure, incapability to respond denotes a poor prognosis with models suggesting median OS of 4.5 months and 11.0 months in higher and lower-risk patients, respectively.7 Therapeutic options following HMA failure remain limited with no standard of care approach. Fortunately, growing understanding of the pathogenesis of MDS and AML have led to the development of a variety of targeted therapies with varying degrees of success in clinical trials. Here, the mechanisms of HMA failure and novel therapeutic options in these patients are reviewed (Table 3).8-55
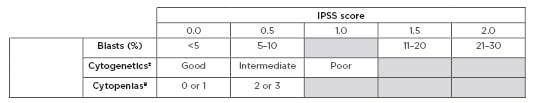
Table 1: International Prognostic Scoring System (IPSS).
±Cytogenetics: good: normal, -Y only, del(5q) only, del(20q) only; Intermediate: abnormalities other than good or poor; Poor: complex >3 abnormalities, chromosome 7 abnormalities.
¥Cytopenias: haemoglobin <10 g/dL; absolute neutrophil count <1,500 cells/µL; platelet count <100,000 /µL.
IPSS risk score interpretation: 0.0 = Low risk 0.5–1.0 = Intermediate-1 risk 1.0–1.5 = Intermediate-2 risk ≥2.5 = High risk
Adapted from Greenberg et al.4
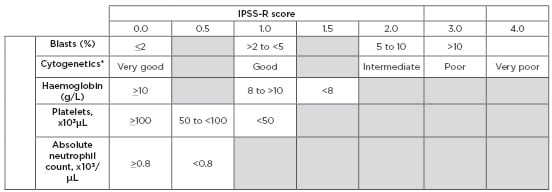
Table 2: Revised International Prognostic Scoring System (IPSS-R).
*Cytogenetics: very good: del(11q) or -Y; Good: normal, del(20q), del(5q), del(12p), or double including del(5q); Intermediate: +8, del(7q), i(17q), +19, or any other single or double independent clone; Poor: -7, inv(3)/t(3q)/del(3q), double including -7/del(7q), or complex (3 abnormalities); very poor: complex >3 abnormalities.
IPSS-R risk score interpretation: <1.5 = Very low risk >1.5 to 3.0 = Low risk >3.0 to 4.5 = Intermediate risk >4.5 to 6.0 = High risk >6.0 = Very high risk
Adapted from Greenberg et al.5
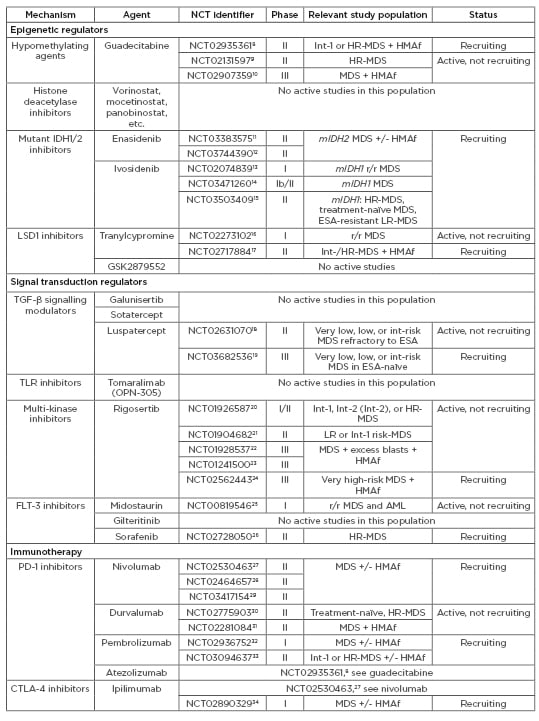
Table 3: Agents under active investigation in patients with myelodysplastic syndromes (MDS).
CTX: chemotherapy; ESA: erythropoiesis-stimulating agents; HMAf: hypomethylating agent failure; HR: high risk; Int: intermediate; LR: low risk; MDS: myelodysplastic syndromes; mIDH: mutant IDH; r/r: relapsed/refractory; TLR: toll-like receptor.
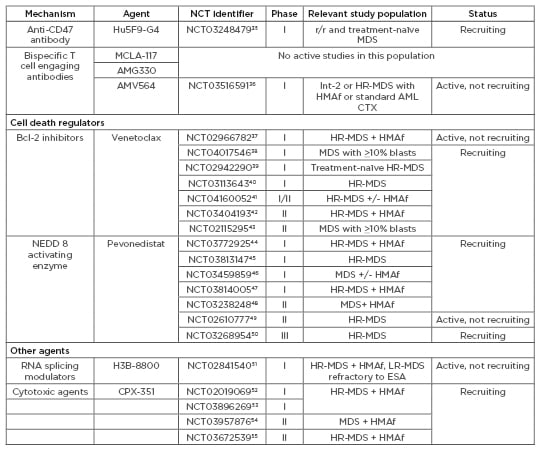
Table 3 continued: Agents under active investigation in patients with myelodysplastic syndromes (MDS).
CTX: chemotherapy; ESA: erythropoiesis-stimulating agents; HMAf: hypomethylating agent failure; HR: high risk; Int: intermediate; LR: low risk; MDS: myelodysplastic syndromes; mIDH: mutant IDH; r/r: relapsed/refractory; TLR: toll-like receptor.
CURRENT THERAPEUTIC OPTIONS FOLLOWING HYPOMETHYLATING AGENT FAILURE
Clinical trials are recommended for patients who fail HMA; however, if not accessible, chemotherapy and haematopoietic stem cell transplant (HSCT) may be used in the appropriate patient.
Cytotoxic Agents and Combinations
Standard AML-based chemotherapy, classically cytosine arabinoside plus anthracycline (7+3), may be used in patients with higher-risk MDS. However, high-dose chemotherapy is associated with prolonged cytopenias with serious infectious complications, and therefore is better tolerated by younger patients with more favourable cytogenetic profiles.2,64 In a larger cohort of 307 patients, of whom 70% were lower risk, comparison of 3 induction regimens (7+3, intermediate versus high dose cytosine arabinoside, or nucleoside analogues) showed similar median OS of 10.8 months, with ORR of 41%, 64%, and 34%, respectively.65 Low-dose chemotherapy (including low-dose cytarabine, hydroxyurea, mercaptopurine, and low-dose melphalan) has not been shown to be superior to best supportive care (BSC), with median OS of 7.3 months.66 However, in a study of predominantly elderly patients with higher-risk MDS after HMA failure, combinations of low-dose cytarabine and clofarabine resulted in a median OS of 10.0 months with 44% ORR.67 Of responders, 30% underwent allogeneic HSCT and 56% achieved long-term remission.
A liposomal formulation of cytarabine and daunorubicin, known as CPX-351, was approved in August 2017 for therapy-related AML and AML with myelodysplasia-related changes.68 Compared to standard 7+3, CPX-351 demonstrated improved OS (hazard ratio [HR]: 0.69; 95% confidence interval [CI]: 0.52–0.90; p=0.005), with a median OS of 9.6 months compared to 5.9 months.68 Given the success of CPX-351, particularly in AML with myelodysplasia-related changes, researchers are investigating the effects of CPX-351 in elderly patients with MDS and AML following HMA failure in a Phase II clinical trial.52
Haematopoietic Stem Cell Transplant
Allogeneic HSCT is the only potentially curative option for patients with higher-risk MDS. However, given its significant toxicity and mortality, use is limited to younger patients or older patients who have a good performance status and donor. Moreover, studies suggest that patients with MDS who failed to respond to HMA have a higher risk of post-HSCT relapse than patients with positive response to HMA.69 In 125 patients treated with HMA, relapse-free survival at 3 years was 23.8% in patients with primary HMA failure and 42.0% among patients who previously responded to HMA.69
EMERGING THERAPIES FOLLOWING HYPOMETHYLATING AGENT FAILURE
Novel Approaches to Hypomethylating Agent Therapy
Despite limited data, sequential use of the alternative HMA as a means of overcoming resistance is not uncommon practice. A few small, mostly retrospective studies have demonstrated between a 19% and 28% response rate to decitabine following azacitidine treatment failure70,71 and 40% to azacitidine following decitabine treatment failure.71
Empiric addition of other agents to HMA in first-line therapy have been studied, including combinations with lenalidomide and vorinostat,72 but have failed to show clinical benefit. Recently, a Phase II study employing a ‘pick a winner’ approach, investigated several combination therapies in patients with higher-risk MDS with the aim of launching more definitive investigations if a successful combination was found.73 Patients were randomly assigned to treatment with azacitidine alone versus azacitidine plus lenalidomide, azacitidine plus valproic acid, or azacitidine plus idarubicin.73 None of these combinations were found to be superior to azacitidine alone.73 Ongoing combination therapies are being evaluated, including a Phase Ib trial investigating azacitidine with venetoclax39 and a Phase III trial of azacitidine with pevonedistat.50 Novel HMA with oral formulations, longer half-lives, and reduced toxicity are also under development.
Oral Hypomethylating Agents
Oral azacitidine and decitabine are currently undergoing evaluation in clinical trials. Compared to traditional parenteral formulations, oral formulations allow for delivery of the drug over a longer schedule and provide convenient dosing schedules for patients. Results from Phase I trials in patients with MDS and chronic myelomonocytic leukaemia (CMML) showed treatment with empiric oral azacitidine yielded an ORR of 73% compared to 35% in patients who were previously treated with traditional injectable formulations.74 Findings from a Phase I study of oral azacitidine in lower-risk patients from this group demonstrated a benefit to extended dosing schedules in which azacitidine given as 300 mg once daily for 14 versus 21 day dosing schedule resulted in an ORR of 36% and 41%, respectively.75 A Phase III trial from this group is ongoing.76
Cedazuridine, a novel cytidine deaminase inhibitor called ASTX727, increases the bioavailability of oral decitabine by inhibiting its degradation in the gastrointestinal tract and liver.77 Phase I studies of oral decitabine in combination with cedazuridine demonstrated similar clinical and biological responses when compared to intravenous decitabine.77 Preliminary results from a Phase II study demonstrated clinical benefit in 62% of patients.78 A Phase III study is ongoing.77
Guadecitabine
Guadecitabine is a novel, second-generation HMA resistant to deamination by cytidine deaminase and therefore exhibits longer half-life than decitabine. Two large, Phase II studies were conducted in patients with intermediate-2 or high-risk MDS and CMML either untreated79 or following HMA failure.80 In the first study, ORR was 61% with a median OS of 15.0 months at a median follow up of 15.0 months.79 The second study, which included patients with HMA failure and treatment-naïve patients, achieved an ORR of 43% and 51%, respectively, with median OS of 12.0 and 23.1 months.80 These results support guadecitabine in first-line therapy. A Phase III trial comparing guadecitabine to standard therapy in patients with MDS and CMML after HMA failure is underway.10
OTHER DRUGS TARGETING EPIGENETIC DYSREGULATION
Histone Deacetylase Inhibitors
One of the key mechanisms in the epigenetic regulation of gene expression is through histone acetylation and deacetylation. Histone deacetylation results in transcriptional deactivation and ultimately downregulation of gene expression. Transcriptional repression complexes, such as histone deacetylases (HDAC), may downregulate tumour suppressor genes.81 Histone deacetylase inhibitors (HDACi) have been assessed for use in MDS alone or in combination with HMA. HDACi may also play a role in apoptosis and induce alterations in the NF-κB pathway.81,82 Despite robust preclinical data and extensive studies in patients with MDS and AML, HDACi have not been shown to improve outcomes in combination therapy with HMA.81,83-85
Mutant IDH1 and IDH2 Inhibitors
Isocitrate dehydrogenases (IDH) are enzymes involved in diverse cellular processes, including histone demethylation and DNA regulation.86 Mutations in IDH1 or IDH2 (mIDH1/2) result in DNA and histone hypermethylation impeding haematopoietic progenitor cell differentiation and promoting leukaemogenesis.86 Together, mIDH1/2 are among the most common mutations in myeloid malignancies, occurring in approximately 20% of patients with AML and 5% with MDS.87 Several mIDH1/2 inhibitors are under development as monotherapy or in combination with HMA or chemotherapy. These include two oral agents recently approved by the FDA for use in AML: enasidenib, a mIDH2 inhibitor approved for relapsed/refractory AML with IDH2 mutation; and ivosidenib, a mIDH1 inhibitor approved for AML with IDH1 mutation.
Enasidenib received FDA approval in August 2017 following a Phase I/II study in relapsed/refractory AML achieving ORR 40.3% with CR 19.3%.88 A significant survival benefit was seen in patients achieving CR with median OS of 19.7 months versus 9.3 months in relapsed/refractory pateints.88 Enasidenib is now being evaluated in patients with MDS after HMA failure, including in two Phase II studies.11,12
Similarly, ivosidenib received FDA approval in July 2018 for relapsed/refractory AML based on results from a Phase I study of 125 patients achieving ORR of 42.0% after 7 months, with CR 22.0% with median duration of 9.0 months. The median OS was 9 months after a median follow-up of 15.0 months.89 Results from an expansion study of 12 patients showed ORR of 91.7%, with median duration 21.4 months and CR 41.7% (median duration not estimable).90 Ivosidenib was recently approved as first-line therapy in AML in May 2019. Ivosidenib is being evaluated in a Phase II study in patients with MDS following HMA failure.15
Lysine Demethylase 1 Inhibitors
Lysine demethylase 1 (LSD1) regulates gene transcription through the removal of methyl groups from histones and is overexpressed in myeloid malignancies. LSD1 inhibitors (LSD1i) have been shown to promote the differentiation of blast cells in AML, particularly in patients with mutations in KMT2A.91 A Phase I/II study of LSD1i GSK2879552 in MDS92 and Phase I study in AML were both terminated early; however, tranylcypromine, another LSD1i, is currently being evaluated in Phase I/II studies with results pending.16,17
DRUGS TARGETING ABNORMAL SIGNAL TRANSDUCTION
TGF-β Receptor Signalling Modulators
Abnormal activation of the TGF-β receptor signalling pathways has been implicated in the pathogenesis of MDS representing a novel therapeutic target, while suppression of this pathway promotes in vitro haematopoiesis in MDS progenitor cells.93
Sotatercept, a selective activin receptor ligand that traps GDF11 to restore effective erythropoiesis, was studied in 74 patients, 48% of whom had HMA failure. 49% of patients achieved erythroid HI, as did 59% of patients with HMA failure and 47% with high transfusion burden.94
Galunisertib, a first-in-class oral inhibitor of the TGF-β receptor type 1 kinase (ALK5), was recently evaluated as monotherapy in 43 patients with very low, low, or intermediate-risk MDS.95 Overall HI was 24.4% (10/41) and 31.1% in transfusion-dependent patients (9/28) with a median response duration of 3 months. Two patients were previously treated with HMA. Although these agents have yet to be tested in higher-risk patients, findings from these studies suggest treatment with sotatercept and possibly other TGF-β receptor signalling modulators may be an option in transfusion-dependent, lower-risk patients following HMA failure.
Toll-like Receptor Inhibitors
Toll-like receptors (TLR) play a key role in innate immune activation through activation of NF-κB. Overexpression of TLR2 on the MDS cell membrane, which is upregulated by HMA therapy and may be implicated in HMA failure, has been shown to inhibit haematopoietic differentiation in MDS.96 Phase I/II studies using tomaralimab (OPN-305), a fully humanised IgG4-κ monoclonal antibody against TLR2, were conducted in patients with lower-risk MDS following HMA failure.96 Preliminary data suggest an ORR of 50%, supporting a role for tomaralimab in the treatment of lower-risk patients with HMA failure.96
Rigosertib Alone and in Combination with Azacitidine
Rigosertib is a multi-kinase inhibitor of cellular signalling through the targeting of the Ras-binding domain of RAS, PI3K/AKT, and RAF/PLK, inducing mitotic arrest and apoptosis in neoplastic cells. Rigosertib is currently undergoing investigation as a single agent in certain subtypes of MDS, including patients with higher-risk MDS following HMA failure, as well as in combination with azacitidine. Although intravenous rigosertib was not shown to improve OS compared to BSC in higher risk patients with HMA failure in the Phase III ONTIME trial, a post hoc analysis of very high-risk patients showed median OS significantly improved to 7.6 months in the study group compared to 3.2 months in BSC.97 Survival benefit was seen in patients with primary HMA failure, monosomy 7 or trisomy 8, and who were <75 years of age.97 A second Phase III study is underway, which will further evaluate these patients with very high-risk disease.24
An oral formulation of rigosertib is also under investigation. Preclinical data demonstrated a synergistic effect with sequential dosing of rigosertib with azacitidine.98 This combination was evaluated in a Phase I/II study of 74 patients with higher-risk MDS.99 A dose of >840 mg per day resulted in ORR 90% and 54% in HMA naïve and HMA failure patients, respectively.99 A Phase III study is anticipated.
FLT3 Inhibitors
The FLT3 gene encodes a tyrosine kinase receptor expressed on haematopoietic progenitor cells, which promotes cellular proliferation and differentiation. FLT3 is mutated in approximately 30% of patients with AML, conferring a poor prognosis with resistance to conventional chemotherapy regimens.100 The FLT3 inhibitors midostaurin and gilteritinib have been approved by the FDA in FLT3-mutated AML, the former in combination with 7+3. Although FLT3 mutations are seen in <1% of patients with newly diagnosed MDS, they are found in up to 5% of patients with MDS transformed to AML.101 Phase I/II studies of the FLT3 inhibitors midostaurin and sorafenib, in combination with azacitidine, demonstrated efficacy with ORR of 26% and 46%, respectively. Although the majority of the study participants had AML, these agents show promise in FLT3-mutated MDS.
IMMUNOTHERAPY
Immune Checkpoint Inhibitors
Immune regulatory proteins PD-1/PD-L1 and CTLA-4 downregulate antitumour T-cell responses and promote tumourigenesis. These immune regulatory proteins were upregulated in MDS cells treated with HMA,102 which may be linked to HMA failure. The PD-1 inhibitor nivolumab, as well as the CTLA-4 inhibitor ipilimumab, were evaluated in a Phase II study in combination with azacitidine in treatment-naïve MDS or as monotherapy in patients with HMA failure.103 In treatment-naïve patients, ORR was 70% with nivolumab/azacitidine and 62% with ipilimumab/azacitidine. Median survival was not reached at a median follow up of 20.1 months in treatment-naïve patients treated with ipilimumab/azacitidine, surpassing the effect of azacitidine alone. Further investigations into these agents, including triple combinations,27,104 are under investigation.
Anti-CD47 Antibodies
Hu5F9-G4 (5F9) is a first-in-class anti-CD47 antibody, which targets a key macrophage immune checkpoint resulting in AML cell phagocytosis.105 Azacitidine enhances phagocytic elimination of AML cells when combined with 5F9.106 A Phase I study of 5F9 alone or in combination with azacitidine in patients with relapsed/refractory AML and MDS among other cohorts is under investigation.35
Bispecific T-cell Engaging Antibodies
Bispecific T-cell engaging antibodies link T cells (via the CD3 receptor) with specific antigens on tumour cells to induce tumour cell apoptosis. An increasing number of tumour-specific antigens are under development, including CD33 and CLEC12A, which are frequently expressed on myeloid precursors in AML and MDS.107,108 Two novel bispecific CD33/CD3 antibodies, AMG330 and AMV564, and a bispecific CLEC12A/CD3 antibody, MCLA-117, are being evaluated in Phase I studies in relapsed/refractory AML.109-111 Preliminary results of AMV564 have been encouraging, with reduction in myeloblasts ranging from 13 to 38% in 6/9 evaluable patients.112 AMV564 is also undergoing investigation in intermediate and high-risk MDS in a Phase I study.36
RNA splicing modulators
Dysregulated mRNA splicing has been implicated in tumourigenesis. Genes involved in spliceosome machinery, including SF3B1, SRSF2, U2AF1, and ZRSR2, are frequently mutated in patients with MDS representing novel therapeutic targets.113 Based on positive results from preclinical trials, a Phase I study of a novel splicing modulator, H3-B8800, is under investigation in MDS patients with HMA failure, CMML, and AML.51
DRUGS TARGETING DEREGULATED CELL DEATH PATHWAYS
Bcl-2 Inhibitors
Bcl-2 is a mitochondrial protein that promotes cellular survival by inhibiting pro-apoptotic pathways. Overexpression of Bcl-2 has been reported in higher-risk MDS leading to resistance of apoptosis114 and to azacitidine.115 Venetoclax is an orally bioavailable potent inhibitor of Bcl-2. In November 2018, venetoclax was approved for older adults with newly diagnosed AML in combination with HMA or low-dose cytarabine who were otherwise not candidates for intensive induction therapy. Interim analysis of a Phase II study42 of venetoclax in combination with decitabine achieved a CR/CR with incomplete blood count recovery of 92% in older patients with newly diagnosed AML, 71% in secondary AML, and 44% in patients with relapsed/refractory AML.116 Given its success in AML, venetoclax is also being investigated in MDS. A Phase Ib study will examine the effect of venetoclax alone or in combination with azacitidine in high-risk patients following HMA failure.37
Pevonedistat
Pevonedistat is a first-in-class inhibitor of the NEDD8 activating enzyme (NAE). NAE is an essential regulator of the degradation of proteins involved in cell cycle progression and cellular stress responses. Preclinical data demonstrated the effect of NAE inhibition in inducing AML cell death117 as well as synergistic effects with azacitidine and decitabine.118 A Phase I study of pevonedistat plus azacitidine in treatment-naïve, older patients with AML showed intention-to-treat ORR of 50%.119 This combination is being evaluated in a Phase II study in patents with MDS after HMA failure.48 The Phase III PANTHER trial is comparing pevonedistat plus azacitidine to azacitidine alone as first-line therapy in higher-risk MDS, CMML, and low-blast AML.50
CONCLUSION
It is critical to recognise HMA failure in MDS because these patients have poor outcomes. Although there are no standard therapeutic options following HMA failure, several emerging therapies with the goal of improving symptom burden and overall survival are showing promise in clinical studies. These include novel targeted therapies and immune therapies to genes commonly altered in MDS. Therefore, clinical trial enrolment is the preferred option after HMA failure. A comprehensive assessment of the patient’s clinical, molecular, and cytogenetic profiles at the time of HMA failure will help guide therapy selection.







