Abstract
Immune thrombocytopenia (ITP) is a common bleeding disorder characterised by isolated thrombocytopenia, due to immune-mediated accelerated platelet destruction, usually without any specific or identifiable precipitating factor. ITP most commonly presents with bleeding associated with a low platelet count <100×109/L. Corticosteroids are the first line of treatment in adults. However, steroid-induced complications are widespread in patients with ITP, and sometimes are more atrocious than the risk of bleeding associated with thrombocytopenia. The authors report the case of a 29-year-old male with ITP with recurrent episodes of epistaxis, who was treated with prednisolone for 8 weeks and developed acne, steroid-induced hyperglycaemia, and urinary tract infection with epididymitis and pyocele. A few weeks later, the patient developed blurring of vision, and was found to have central serous chorioretinopathy. They were treated adequately for each of these complications, and had complete resolution of symptoms following cessation of steroids. While acne and hyperglycaemia are common, urinary tract infections with epididymitis and central serous chorioretinopathy are infrequent complications following steroid administration. The complexity of adverse events and the challenges in diagnosing and treating these unique complications prompted the authors to report this case.
Key Points
1. This article summarises the occurrence of very unusual and rare steroid-related toxicity. It is a cautionary tale of modern medicine where the treatment-related adverse events are more atrocious than the primary disease.
2. This article highlights the importance of close monitoring of patients on steroids, which would help detect any steroid-related toxicity early in the course of occurrence.
3. Early intervention and timely treatment can revert the toxicities of steroid therapy, and
prevent the advent of permanent organ damage. Further, this case also teaches that patients with immune thrombocytopenia may be considered for shorter duration of steroid therapy, and steroid sparing agents should be considered early, especially in those prone to develop therapy-related adverse events.
INTRODUCTION
The aetiology of immune thrombocytopenia (ITP) is a subject of an enigma, and the diagnosis is one of exclusion.1 The standard recommendation is to initiate therapy in an adult with newly-diagnosed ITP with a platelet count <3×109/L, regardless of any bleeding manifestations.2,3 Bleeding in ITP may range from mild petechial spots to severe intracranial bleeds.4,5 The severity of bleeding rarely correlates with the severity of thrombocytopenia. Corticosteroids remain the most elementary group of drugs in the initial management of ITP, alongside intravenous immunoglobulin and anti-D immune globulin (in the Rhesus-positive blood group).2 Systemic corticosteroids are administered at a high dose (prednisolone 1 mg/kg) for more extended periods in treating ITP, owing to the relapsing and remitting course of the disease.6,7 This may lead to a multitude of adverse events.8,9
The authors report a case of a young male with newly-diagnosed ITP. The patient was started on corticosteroids and developed several steroid-related complications, including hyperglycaemia, acne, and urinary tract infection (UTI). They also complained of blurred vision, and were diagnosed with central serous chorioretinopathy (CSC). They were treated adequately for each of these complications, and recovered completely. While steroid-induced hyperglycaemia and acne are quite common, UTIs in males and pyocele are rare complications associated with steroid administration. CSC in patients with ITP following short-course steroids is singularly uncommon. This case report highlights the importance of close monitoring of patients on steroids, and the significance of timely intervention in treating therapy-related complications, and thereby preventing permanent end-organ damage and morbidity.
CASE REPORT
A 29-year-old male presented to the authors’ outpatient department with a history of three episodes of acute onset epistaxis (World Health Organization [WHO] Bleeding Score: Grade 2)10 over 1 week. The patient had no history of bleeding in the past; nor was there any history of bleeding among their siblings or family in the past. The patient was conscious and alert on examination, and their vitals were stable. There was no pallor, icterus, cyanosis, clubbing, or pedal oedema. The patient had left-sided scrotal swelling. There was no pain, redness, or tenderness over the swelling. The rest of their systemic examination was unremarkable. The complete blood count showed isolated thrombocytopenia viz haemoglobin 11.7 g/dL, total white blood cell (WBC) count 5×109/L, and platelet count 5×109/L with a differential WBC count of 60% neutrophils, 34% lymphocytes, and 6% monocytes. Peripheral smear examination showed microcytic to macrocytic red blood cells with moderate anisocytosis, adequate WBCs, and markedly reduced platelets. Other investigations included serum electrolytes, urea and creatinine, serum bilirubin and transaminases, HbA1c, serum lactate dehydrogenase, erythrocyte sedimentation rate, C-reactive protein, thyroid function test, serum antinuclear antibody, urine examination, and chest X-ray; all were normal. Screening for blood-borne viral diseases such as HIV, hepatitis C virus, and hepatitis B surface antigen were negative. Direct and indirect antiglobulin test was negative. The patient’s baseline abdomen, pelvis, and scrotum ultrasonogram showed a left-sided hydrocele.
Since all the possible secondary causes of thrombocytopenia were ruled out, the patient was diagnosed with ITP and started on tablet prednisolone at a daily dose of 1 mg/kg. They were followed up weekly to monitor their platelet count, and to look for any possible steroid-related complications. The patient developed hyperglycaemia within 1 week of starting steroids, and was treated with oral hypoglycaemic agents. An adequate glycaemic control was achieved with oral hypoglycaemic agents, without the requirement of insulin. Therefore, steroids were continued along with oral hypoglycaemic agents. The patient developed papules and comedones on their face and trunk over the next few days, i.e., within 1 week of starting steroids, and was treated for acne with adapalene and benzoyl peroxide ointment. Their acne improved significantly following topical therapy.
The patient had a partial haematological response to steroids with a rise in the platelet count to 70×109/L at the end of 4 weeks of steroid therapy. So, prednisolone was tapered. However, following the initiation of tapering of steroids at 4 weeks of therapy completion, there was a sudden drop in platelet count to 16×109/L, and the patient developed one episode of epistaxis (WHO Bleeding Score: Grade 2)10. Since the patient had a recurrence of bleeding along with a drop in platelet count at the time of steroid tapering, the prednisolone dose was increased again, and a very slow tapering of prednisolone dose was planned. Around 6 weeks of steroid therapy, the patient developed a burning micturition. A routine and microscopic examination of urine showed the presence of numerous red blood cells and pus cells, and urine culture sensitivity showed the growth of Klebsiella species (Table 1). The patient was started on oral antibiotics, anti-spasmodic agents, and supportive measures. They also complained of pain and swelling over the left scrotum. Clinical examination of the left scrotum showed inflamed skin over the left-sided hydrocele, and there was significant tenderness on palpation of the swelling. An urgent scrotum ultrasound was done, which revealed an oedematous left epididymis and increased echogenicity, with internal echoes of the left-sided scrotal swelling suggestive of left-sided epididymitis and pyocele. An urgent urology opinion was sought, and steroids were rapidly tapered and stopped. The patient’s expressed prostatic secretion was sent for culture and sensitivity, and showed the growth of Klebsiella species (Table 1). The patient was treated with appropriate antibiotics as per the culture and sensitivity report for a total duration of 2 weeks. They were also treated with other supportive measures, such as analgesics, scrotal support, and anti-spasmodic agents. They had a spontaneous rupture of the pyocele with a spontaneous pus drainage, obviating the need for surgical intervention. They had remarkable symptomatic relief and complete resolution of epididymitis and pyocele. A repeat scrotum ultrasound done after 2 weeks of antibiotic therapy showed normal bilateral scrotum, epididymis, and normal bilateral testicles.
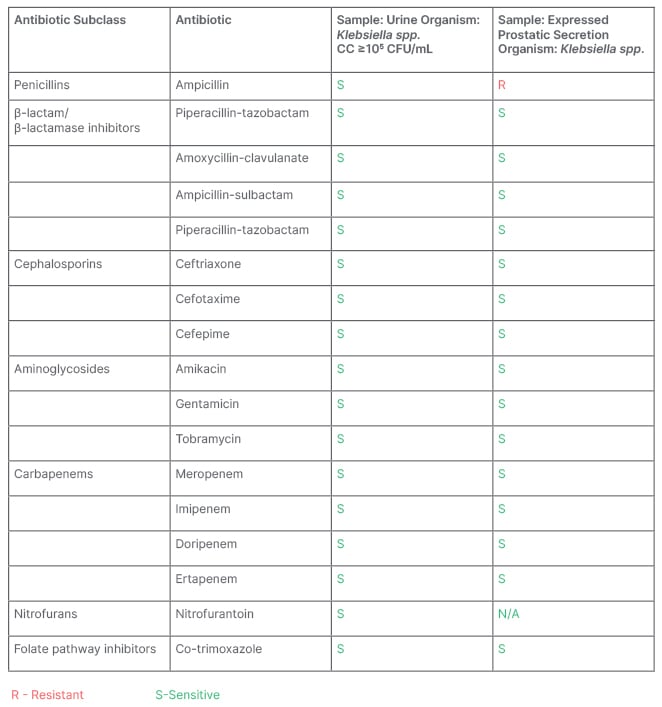
Table 1: Urine and expressed prostatic secretion culture and sensitivity.
CC: colony count; CFU: colony forming unit; Klebsiella spp.: Klebsiella pneumoniae; N/A: not applicable.
Following this, the patient was on a regular follow-up to monitor their platelet count and assess their wellbeing as steroids were stopped. Their platelet count was 70×109/L at 8 weeks of therapy completion. However, the patient complained of blurred vision in both eyes around this time. They were off steroids at this point. A detailed ophthalmologic evaluation revealed a best-corrected visual acuity (BCVA) of 20/40 and 20/20 in the right eye and left eye, respectively. The anterior segment examination of both eyes was unremarkable. Intraocular pressures of both eyes were also within normal limits. Fundus evaluation of the right eye showed a dull foveal reflex with subretinal fluid and multiple yellow-pigmented spots in the foveal region. In contrast, the left eye had a normal foveal reflex. Further evaluation with a spectral domain optical coherence tomography (OCT) confirmed the presence of subretinal fluid (SRF) in the fovea. It revealed pigment epithelial detachments with subretinal deposits in the right eye (Figure 1A, 1C, 1D), and a normal fovea of the left eye (Figure 1B). A diagnosis of steroid-induced CSC was made. The patient was advised close observation, and was kept on a regular follow-up. Repeat OCT done after 2 weeks showed a reduction in SRF and improved BCVA to 20/30 in the right eye. After a follow-up of 1 month, BCVA was restored to 20/20 in the right eye (Figure 1E) with a complete resolution of subretinal fluid, and the left eye continued to be normal (Figure 1F).
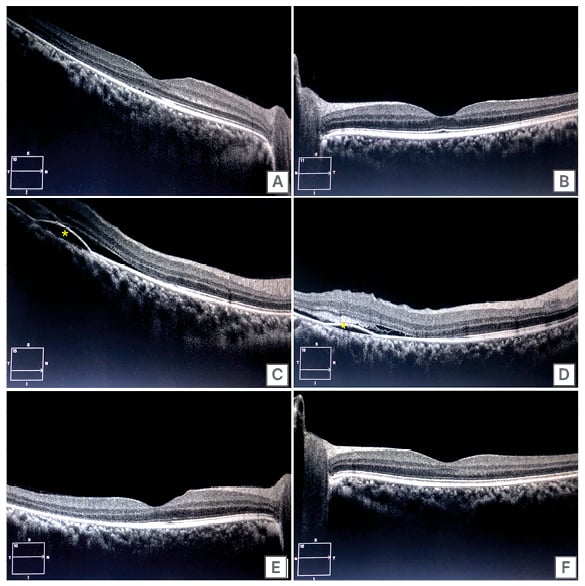
Figure 1: Optical coherence tomography images.
A) OCT of the right eye showing minimal subfoveal neurosensory detachment; B) OCT of the left eye
showing a normal foveal contour with intact retinal layers; C) OCT of right eye with a section inferior to fovea demonstrating PED (yellow asterisk), neurosensory detachment with shallow SRF; D) OCT of right eye with another section inferior to fovea demonstrating PED, SRF, and subretinal hyperreflective material (yellow arrow); E) OCT of right eye after 1 month showing resolution of SRF and a normal foveal contour; F) Left eye continued to be normal after 1 month.
OCT: optical coherence tomography; PED: pigment epithelial detachment; SRF: subretinal fluid.
Further, on follow-up, the patient’s platelet count was 40×109/L approximately, while not on any therapy, and they had no bleeding manifestations. Hence, no second-line treatment was started, and the platelet count was closely monitored. It was decided to initiate a second line of therapy should there be any decrease in platelet count to <20×109/L, or any new onset bleeding manifestation.
DISCUSSION
ITP is a common bleeding disorder characterised by isolated thrombocytopenia without any specific or identifiable precipitating factor.11 The decreased platelet count is usually secondary to immune-mediated accelerated platelet destruction and decreased platelet production.12 ITP may occur without any apparent underlying cause (primary) or associated with other underlying diseases (secondary ITP). Secondary causes include infections (both bacterial [Helicobacter pylori] and viral, including varicella zoster, hepatitis C virus, and HIV viral infections), autoimmune diseases, underlying lymphoproliferative disorders, drugs, etc.11
Bleeding in ITP is heterogenous, unpredictable, and most likely associated with many environmental and genetic risk factors.13 The patient with ITP may be asymptomatic, or present with mild mucocutaneous or severe life-threatening bleeding.4,13 The incidence of extreme life-threatening bleeding in ITP is fortunately low, approximately 9.6% and 20.2% in adults and children, respectively.2 Though severe bleeding is rare, it is the most critical clinical endpoint, and crucial indication to initiate therapy.
In addition to the bleeding manifestations, patients with ITP also present with fatigue, anaemia, and poor health-related quality of life. A final rationale for treating an asymptomatic patient with ITP upfront is to prevent chronic and relapsing disease.2,14 The standard recommendation is to initiate therapy in an adult with newly-diagnosed ITP with a platelet count <3×109/L, regardless of the initial bleeding manifestations. The International Working Group (IWG) devised the ITP-specific bleeding assessment tool (ITP–BAT) to allow for uniformity of assessment of bleeding manifestations. However, the simplicity of application makes the WHO bleeding score10 a more widely used bleeding assessment tool.
Corticosteroids remain the most elementary group of drugs in the initial management of ITP.11,15 Intravenous immunoglobulin and anti-D immune globulin (in the Rhesus-positive blood group) are also recommended as first-line therapy.3,11 Second- and third-line treatment options are generally reserved for those who fail first-line therapy. These therapeutic strategies include immunomodulators, such as rituximab, azathioprine, and dapsone, and thrombopoietin-stimulating agents like eltrombopag and romiplostim.6,16
Glucocorticoid treatment is the standard initial therapy in ITP because of its effectiveness in increasing the platelet count, low cost, and convenience. Approximately 60–80% of patients with ITP have an initial response to glucocorticoids, but only 30–50% of adults have a sustained response after it is discontinued.7 For most patients, the platelet count response only lasts as long as the corticosteroids are continued. The duration of corticosteroid treatment is not standardised; it is often continued for 3–4 weeks, or until side effects become intolerable.17 Long-term steroid administration is associated with a myriad of complications. While hyperglycaemia, acne, and infections are the more common complications, CSC is a relatively rare entity.8
Steroid-induced hyperglycaemia causes new-onset hyperglycaemia or worsening glucose control in patients with previously known diabetes,18 with an incidence of 46% in patients with diabetes and 68% in patients with increases in glucose levels compared to baseline,18-20 making it one of the commonest steroid-related toxicities. Furthermore, there may be acute complications such as a nonketotic hyperosmolar state or diabetic ketoacidosis. No evidence exists to establish therapeutic goals for patients with steroid-induced hyperglycaemia. According to the American Diabetes Association (ADA), glucose targets should be individualised according to specific factors, such as life expectancy, comorbidities, patient compliance, and risk of hypoglycaemia. In hospitalised patients, a target glucose range of 140–180 mg/dL is recommended for critically and non-critically ill patients. More stringent goals, such as 110–140 mg/dL, may be appropriate for selected patients if this goal can be achieved without hypoglycaemia. Oral hypoglycaemic agents might suit inpatients with stable and non-critical disease and mild hyperglycaemia.18,21 In contrast, for those with significant hyperglycaemia and severe illness, insulin remains the treatment of choice. The authors’ patient was treated with oral hypoglycaemic agents, and achieved optimal glycaemic control.
Acneiform lesions in steroid acne are usually seen on the chest, but may also be seen on the face, neck, back, and arms. In most cases, steroid acne resolves with the discontinuation of steroid therapy. However, in clinical scenarios where prolonged steroid administration is warranted, other modalities may be used, including topical preparations like salicylic acid and benzoyl peroxide; retinoids; and topical antibacterial or antifungal therapy, like doxycycline and ketoconazole.22,23 The authors’ patient was treated with retinoid (adapalene) and benzoyl peroxide, and improved significantly.
UTIs are among the most common ailments, affecting 150 million people worldwide yearly.24 However, UTIs in young males are very unusual, and increase dramatically with age.25 Urethritis and epididymitis are painful conditions caused by bacterial infections of the urethra and epididymis, respectively. Acute epididymitis is usually treated in the outpatient setting. Rarely, intravenous antibiotics are required for systemic symptoms, abscess formation, or Fournier’s gangrene. Epididymitis usually improves within 2–3 days of antibiotic treatment, but residual pain may persist for several weeks. In addition, supportive therapy in the form of analgesics, anti-inflammatories, and scrotal elevation is recommended for acute epididymitis.26
The authors’ patient developed a complicated UTI with an infected hydrocele (pyocele), left-sided acute epididymitis, and urethritis. There was a spontaneous rupture of the pyocele. The patient was treated with oral antibiotics for 2 weeks and other supportive measures, and had a complete resolution of symptoms following treatment.
CSC is a chorioretinal disease that causes an idiopathic serous retinal detachment. It is associated with one or more areas of leakage from the choroid through a defect in the retinal pigment epithelium outer blood–retina barrier.27 Most patients are ales who have decreased and distorted vision together with altered colour appreciation.28 The exact mechanism of steroid-induced central serous retinopathy is not very well defined. The pathophysiology of CSC is multifactorial, which causes retinal pigment epithelial disturbances and circulatory changes in the choroid. Although the natural history of CSC shows a self-limiting course, patients are known to present with persistent, recurrent, or even bilateral CSC with distressful visual loss. OCT is the first line of investigation in CSC. The presence of SRF is characteristic of CSC. The resolution of SRF can be documented on serial OCT.29 Corticosteroid use is the most critical identifiable external risk factor in patients with CSC.30 The primary approach to treating CSC is conservative with observation, and requires cessation of steroid intake.
Burkhodari et al.31 and Sandhya et al.32 also demonstrated a similar occurrence of central serous retinopathy in ITP, but without complete vision recovery. There is no gold standard for the treatment of persistent CSC. The U.S. Food and Drug Administration (FDA) approves no therapeutic options. However, local modalities, both pharmacologic and photic, and systemic medical treatments are under ongoing investigation. They may hold promise for future patients diagnosed with CSC.27,33,34
The authors’ patient rapidly recovered normal vision in both eyes within 1 month of stopping steroids. Other complications, including steroid-induced hyperglycaemia, acne, and UTIs, also resolved with treatment. Figure 2 depicts a graph representing the temporal association of events.
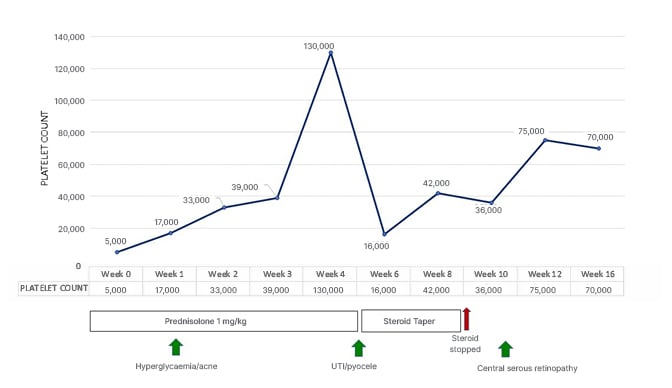
Figure 2: Graph depicting the temporal association of events.
CONCLUSION
The authors conclude that patients on systemic steroids for ITP, or any other systemic illness, should be closely monitored for the development of any steroid-related adverse event. The occurrence of a chain of steroid-related complications in succession, including hyperglycaemia, acne, UTIs, pyocele, and the delayed onset of central serous retinopathy, even after cessation of steroid therapy, prompted the authors to report this case. This case emphasises the role of close follow-up and vigilant monitoring, which helped in the early detection of complications. This case also taught the authors as a team that considering a shorter duration of steroid therapy, early initiation of steroid-sparing second-line therapy, and a closer follow-up in cases of patients prone to steroid toxicity, together with timely intervention and optimal treatment, can prevent the occurrence of such series of adverse events in future. The authors hope to re-emphasise and create a more comprehensive awareness of the atrocities of steroid-related toxicity so that physicians may consider curtailing the dose and duration of steroid therapy, and prevent the advent of permanent organ damage.






