BACKGROUND
Understanding how loss of function mutation of TET2 in haematopoietic stem cells (HSC) changes their response to stress is crucial to elucidating the dynamics of clonal haematopoiesis throughout our lifespan. Over time, as we age, we encounter episodes of infection and hematopoietic stresses, yet how these complex episodes influence the expansion of the mutant clones needs to be fully understood. Previous work in mouse models has delineated the contribution of specific proinflammatory cytokines in the competitive advantage of TET2-KO HSCs;1-3 but the impact of multifactorial episodes, such as viral or bacterial infections, into the clonal evolution at the human cell level is scarce. Characterising when and how TET2 mutant human HSCs are favoured could help design therapeutic strategies to reduce the expansion of clonal haematopoiesis (CH) and the risk of individuals with CH developing haematological diseases and inflammatory pathologies.
AIMS
To provide new evidence of how TET2-derived clonal haematopoiesis evolves during our lifespan, and which environmental cues are more likely to favour TET2 mutant HSCs.
METHODS
The authors introduced CRISPR TET2 mutations into primary human HSCs (hHSC), allowing for a comprehensive examination of their behaviour under stress conditions. First, the authors characterised the response of TET2-mutant hHSCs and differentiated progeny upon different immune challenges in long-term culture assays. The authors reproduced a TET2-derived clonal haematopoiesis scenario in humanised mice,4 and performed functional, transcriptomic, and epigenomic analyses within the haematopoietic stem and progenitor cells (HSPC) compartment.
RESULTS
The authors unveil that hHSCs carrying a loss of function (Lof) TET2 mutations exhibit heightened resistance to haematopoietic stress compared to their wild-type counterparts. Interestingly, using long-term culture assays (Figure 1A), the authors tested the behaviour of TET2-mutant HSCs under different sources of stress (Figure 1B), and identified lipopolysaccharide (LPS) as the immune threat that leads to higher expansion of mutant clones (Figure 1C). Bulk RNA sequencing was performed to analyse cell-specific transcriptional response and the impact of TET2 LoF on LPS in both primitive CD34+ cells and differentiated myeloid CD14+ cells (Figure 1D). Interestingly, while TET2-mutant HSCs have a mitigated transcriptional upregulation of inflammatory pathways upon LPS response (Figure 1E), TET2-mutant myeloid cells showed an exacerbated inflammatory response (Figure 1F), consistent with previous reports characterising the impact of TET2 mutations in human myeloid progeny.4 The refractoriness of TET2-mutant HSC to LPS-induced stress was further validated by the increased long-term culture-initiating cell frequency (Figure 1G). Furthermore, employing a humanised mice model, transplanted with a mixture of wild-type and TET2 HSPCs, with or without LPS treatment (Figure 1H), and subsequently investigating the transcriptomic profile of the human HSPC compartment present in these mice by single-cell RNA sequencing, the authors identified two distinct subsets of HSCs within the human HSC pool characterised by differing properties in their response to haematopoietic stress (Figure 1I).
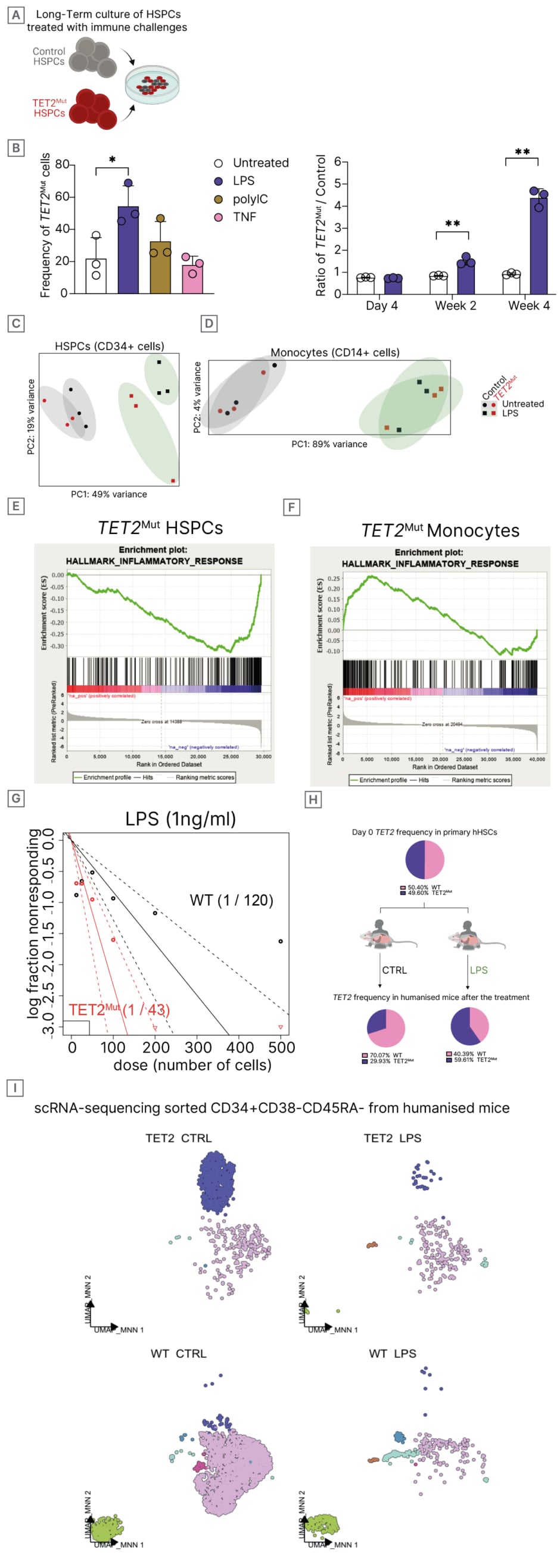
Figure 1: Opposite and cell-specific impact of TET2 mutations in haematopoietic stem cells and myeloid progeny to inflammatory stress.
* P value <0.05 and ** P value <0.01 using Anova test. CTRL: control; HSPCs: haematopoietic stem and progenitor cells; LPS: lipopolysaccharide; MNN: Mutual Nearest Neighbours; PC: principal component; PolyIC: polyinosinic:polycytidylic acid; TNF: tumor necrosis factor; WT: wild-type; UMAP: Uniform Manifold Approximation and Projection.
Notably, within the subset that retained high HSC signature, while wild-type HSCs show a clear activation of transcriptional pathways, TET2 mutant HSCs show no significant changes in transcriptional programmes associated with LPS response.
DISCUSSION
The authors’ work underscores the cell-specific influence of TET2 mutations on the composition and functionality of HSCs and their immune progeny. This study showed the opposite consequence of the TET2 Lof between HSC and myeloid progeny upon an inflammatory challenge. TET2 mutant myeloid cells show exacerbated production of inflammatory cytokines, which is consistent with previous reports.4-8 In sharp contrast, TET2 mutant HSCs show a protective behaviour to LPS-mediated stress. Therefore, this cell-specific TET2-mediated phenotype provides novel perspectives into the clonal haematopoiesis dynamics upon environmental stress.






