Abstract
Gastroesophageal reflux disease (GERD) is one of the most commonly encountered gastrointestinal diseases in clinical practice. Proton pump inhibitors (PPI) remain the cornerstone of the treatment of GERD. Up to one-third of patients do not respond to optimal doses of PPI and fall into the category of refractory GERD. Moreover, the long-term use of PPI is not risk-free, as previously thought. The pathophysiology of refractory GERD is multifactorial and includes reflux related and unrelated factors. It is therefore paramount to address refractory GERD as per the aetiology of the disease for optimal outcomes. The management options for PPI refractory GERD include optimisation of PPI, lifestyle modifications, and the addition of alginates and histamine-2 receptor blockers. Neuromodulators, such as selective serotonin reuptake inhibitors or tricyclic antidepressants, may be beneficial in those with functional heartburn and reflux hypersensitivity. Laparoscopic antireflux surgeries, including Nissen’s fundoplication and magnetic sphincter augmentation, are useful in patients with objective evidence of GERD on pH impedance studies with or without a hiatal hernia. More recently, endoscopic antireflux modalities have emerged as an alternative to surgery in patients with PPI-dependent and PPI-refractory GERD. Long-term data and randomised comparison studies, however, are required before incorporating endoscopic therapies in the management algorithm for refractory GERD.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common gastrointestinal (GI) disorder worldwide. Proton pump inhibitors (PPI) are the mainstay of treatment for GERD and achieve symptom relief in the vast majority of patients. However, approximately one-third of patients continue to experience symptoms of GERD on once daily PPI therapy and 10% of patients on twice daily PPI therapy.1,2 While on long-term PPI therapy, approximately 50–60% of patients complain of persistent or breakthrough symptoms.3 Refractory GERD is associated with poor health-related quality of life (HRQoL), low work productivity, greater absenteeism from work, psychological impairment, and poor sleep quality.4 Moreover, recent studies and guidelines report significant health risks associated with long-term PPI therapy.5 Considering the significant burden of GERD on the healthcare system and a sizeable proportion of patients with suboptimal response to medical therapy, it is crucial to understand novelties in the field of refractory GERD. In this review, the authors discuss the pathophysiology, diagnosis, and various treatment options for PPI refractory GERD.
DEFINITION
Heartburn and regurgitation are the two cardinal symptoms of GERD. The most accepted and pragmatic definition of refractory GERD is the persistence of typical symptoms, heartburn and/or regurgitation, that do not respond to a standard double dose of PPI for a treatment period of at least 12 weeks.2 In clinical practice, the diagnosis of refractoriness can usually be established after 8 weeks of PPI therapy.
Pathophysiology of Refractory Gastroesophageal Reflux Disease
The causes of refractory GERD can be classified as reflux related and nonreflux related (Table 1). In the majority of the cases, refractoriness to PPI can be attributed to residual acid reflux, nonacidic or weakly acidic reflux, acid pocket, oesophageal hypersensitivity, and functional heartburn.
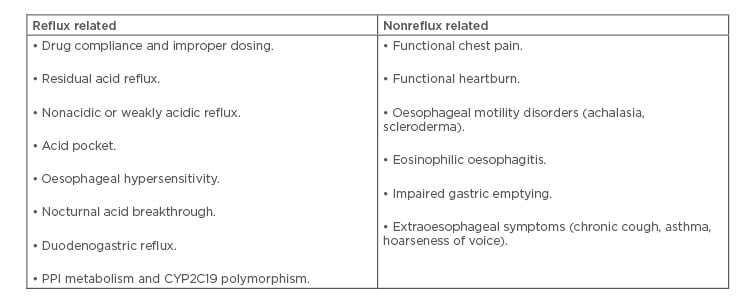
Table 1: Causes of proton pump inhibitor refractory gastroesophageal reflux disease.
CYP2C19L: cytochrome P450 2C19; PPI: proton pump inhibitor.
Drug Compliance and Improper Dosing
One of the most important factors that contributes to refractory GERD is poor drug compliance and improper dosing. In a large population-based survey, strict adherence to PPI treatment was observed in only 38.7% and 30.6% of patients over 6 months and 1 year, respectively.6 Improper timing of PPI intake is another important factor resulting in suboptimal acid suppression and refractoriness to PPI. Administration of PPI 30–45 minutes before a meal is crucial because gastric proton pumps are activated following food intake; however, there is no systematic study or direct evidence to suggest symptom improvement with strict drug adherence.
Weakly Acidic or Nonacidic Reflux
Weakly acidic reflux is defined as a fall in oesophageal pH by at least 1 unit; however, the pH remains between 4–7 and does not fall below 4. A pH cut-off value of 7 is used to differentiate between weakly acidic and nonacidic reflux.7 In some studies, nonacidic reflux episodes have been found in 60–80% of patients with symptoms on double dose PPI therapy.1,8 The proposed mechanisms of symptom generation include stretching of the oesophageal wall attributable to volume reflux, and sensitisation of the oesophagus because of increased acid exposure.9,10 In addition, duodenogastroesophageal reflux may generate symptoms in a small subset of patients by impairing oesophageal mucosal integrity, producing dilated intercellular spaces, and even inducing epithelial apoptosis.11
Acid Pocket
The acid pocket is an area of unbuffered acid compartment that forms in the proximal part of the stomach after meals and serves as a potential reservoir for acid reflux in healthy subjects as well as GERD patients. Patients with GERD are predisposed to upward migration of proximal margin of the acid pocket when compared with healthy controls.12 The acid pocket is also a potential therapeutic target and can be attenuated by PPI or alginates that form a pH neutral raft at the gastroesophageal junction and displace the acid pocket distally.13
Nocturnal Acid Breakthrough
Nocturnal acid breakthrough (NAB) is defined as the persistence of intragastric pH <4 for >60 minutes during the night. NAB was initially thought to be responsible for PPI refractory symptoms and could be abolished by either doubling the dose of PPI or adding an H2 receptor antagonist at night.14 However, the clinical significance of NAB is not clear and some of the latter studies revealed that oesophageal acid reflux and symptoms were independent of the occurrence of NAB.15,16
Protein Pump Inhibitor Metabolism and CYP2C19 Polymorphism
PPI are predominantly metabolised in the liver by CYP2C19 and, to a lesser extent, CYP3A4. Rapid metabolisers show decreased gastric acid suppression with PPI, which may result in reduced efficacy and sustained symptomatic response compared to intermediate or poor metabolisers.17
Functional Heartburn
Functional heartburn is defined as burning retrosternal discomfort or pain that is refractory to optimal antisecretory therapy in the absence of objective evidence of GERD or major oesophageal motility disorder. Nearly one-third of the patients with reflux symptoms may have functional heartburn.18,19 These patients are more refractory to antireflux therapy than those with erosive oesophagitis, and may have other coexistent functional disorders such as functional dyspepsia or irritable bowel syndrome.20-22
Oesophageal Hypersensitivity
Oesophageal hypersensitivity is defined as the presence of typical reflux symptoms without evidence of pathological reflux on endoscopy or pH impedance monitoring, but with demonstration of positive symptom correlation with physiological reflux. Oesophageal hypersensitivity may contribute to symptoms in approximately 30–35% of patients with nonerosive reflux disease.23 Various pathophysiological mechanisms of symptoms have been proposed in these patients including peripheral or central sensitisation, altered central processing of peripheral visceral stimuli, autonomic and psychological abnormalities, and increased permeability of oesophageal mucosal barrier.24,25 Upregulation of acid sensitive receptors, such as TRPV1 and protease-activated receptor 2, has been demonstrated in patients with oesophageal hypersensitivity.26
Eosinophilic Oesophagitis
The symptoms of eosinophilic oesophagitis, including heartburn, chest pain, and dysphagia, can mimic those of GERD; however, the actual prevalence of eosinophilic oesophagitis is very low among patients with refractory GERD.27 Therefore, an oesophageal biopsy may be cost-effective if the prevalence of eosinophilic oesophagitis is high (>8%) in the general population.28
DIAGNOSIS
A detailed evaluation of refractory GERD includes a thorough clinical evaluation, upper GI endoscopy, oesophageal motility assessment using high definition manometry, and reflux monitoring, preferably with a multichannel intraluminal pH-impedance monitor.
Clinical Evaluation
The following details should be obtained from the patient’s history and physical examination:
- Presence of typical and atypical symptoms of GERD.
- Proper dosing and timing of PPI.
- Presence of other functional GI disorders such as functional dyspepsia and inflammatory bowel syndrome.
- Time of occurrence of troublesome symptoms i.e., nocturnal or post meals.
- Presence of alarming symptoms such as weight loss, anorexia, dysphagia, odynophagia, and upper GI bleeding.
- Coexistence of psychological comorbidity.
Endoscopy
Patients with typical GERD symptoms who fail to respond to initial PPI therapy despite optimisation of dose should undergo an upper GI endoscopy. Unfortunately, the diagnostic yield of endoscopy in refractory GERD is limited.29 In the absence of erosive oesophagitis, random oesophageal biopsies can be obtained to examine dilated intercellular spaces and to rule out eosinophilic oesophagitis. The presence of dilated intercellular spaces and higher intercellular space diameter favours nonerosive reflux disease (NERD) and oesophageal hypersensitivity over functional heartburn.30 Narrow-band imaging (NBI) with magnification endoscopy may help in identifying ongoing acid reflux in patients with NERD in the absence of obvious erosions or ulcerations on white light endoscopy. Increased and dilated intrapapillary capillary loops at the lower oesophagus, increased vascularity at the squamo–columnar junction, and tubular and villous pit patterns below the Z line are observed with NBI, and are more frequently seen in NERD compared to controls.31
Reflux Monitoring
Patients who are refractory to PPI therapy and have a normal endoscopy should undergo reflux monitoring. Currently, there are four available options for reflux monitoring: 1) catheter-based pH monitor; 2) wireless capsule pH assessment (Bravo™, Medtronic plc, UK); 3) combined multichannel intraluminal pH impedance monitor; and 4) oesophageal Bilitec™ (Bilitec™ 2000, Medtronic plc, Denmark). Extended recording times (48–96 hours) using wireless pH recording systems increase the diagnostic yield and may be especially useful in those with high suspicion of GERD but negative pH results. The commonly measured variables using pH testing include acid exposure time, number of reflux episodes, symptom index, and symptom association probability. Acid exposure time is qualified as normal (<4%), abnormal (>6%), and inconclusive (4–6%). Similarly, number of reflux episodes are classified as normal (<40/ 24 hours), abnormal (>80), and inconclusive (40–80). The decision to perform pH-monitoring ‘off’ or ‘on’ PPI is dependent upon the clinical scenario. Reflux monitoring ‘off’ PPI is performed when the diagnosis of GERD is unproven with no prior positive pH testing, or preoperatively before definitive surgical or endoscopic antireflux therapy. Whereas, reflux monitoring ‘on’ PPI is performed in patients with proven GERD in form of severe oesophagitis, long segment Barrett’s, and prior abnormal pH result. These patients are typically on double dose PPI and pH-impedance testing is meant to establish the relation between reflux episodes and symptoms.
Nonacidic reflux, rather than acid reflux, appears to be the main driver of symptoms in PPI refractory cases.32 Therefore, the diagnostic yield of traditional catheter-based or wireless (Bravo) pH monitoring is limited in PPI refractory GERD patients because of their inability to measure nonacidic reflux.33 Combined multichannel intraluminal impedance pH monitoring is considered as the gold standard in the evaluation of PPI refractory GERD.34
Symptom association metrics including symptom index and symptom association probability provide analysis of temporal association between symptom occurrence and reflux episodes, and help in assessing the cause of patients’ symptoms. These parameters have major clinical implications in diagnosis and prediction of response to medical and surgical antireflux therapy. In the absence of pathological reflux, a positive symptom index (>50%) and symptom association probability (>95%) suggests oesophageal hypersensitivity. On the other hand, a negative symptom association implies functional heartburn.35
In addition to the above parameters, two novel impedance metrics, post reflux swallow induced peristaltic wave (PSPW) and mean nocturnal baseline impedance (MNBI), improve the diagnostic yield of pH-impedance testing and differentiate patients with pathological reflux from those with functional heartburn. The PSPW index is the proportion of reflux episodes followed by a PSPW and is a marker of integrity of primary peristalsis and oesophageal contraction reserve. A lower PSPW index helps to differentiate erosive oesophagitis from functional heartburn with high sensitivity (99–100%) and specificity (92%).36 Similarly, low MNBI has been reported in erosive oesophagitis, PPI responsive NERD, and oesophageal hypersensitivity compared to PPI refractory cases and functional heartburn.37-39 In addition, low MNBI (<2,292 ohms) independently predicts symptomatic improvement following antireflux surgery.40
Oesophageal Manometry
Oesophageal high-resolution manometry is performed to localise the lower oesophageal sphincter (LES) before placement of a pH probe. In addition, the assessment of oesophageal peristaltic function using the distal contractile index is crucial before definitive antireflux surgery. Up to 40% of patients with preoperative ineffective oesophageal motility (IEM) (distal contractile index <450 mmHg/cm/s in >50% swallows) might experience postoperative dysphagia.41 In patients with IEM, multiple rapid swallows (MRS) can help further identify contraction reserve in the oesophagus. Absence of post MRS augmentation in patients with IEM predicts poor response to prokinetic drugs, higher oesophageal acid exposure in NERD, and dysphagia following antireflux surgery.42 The Lyon consensus recently proposed routine incorporation of MRS into HRM protocols for establishing the contraction reserve in IEM, especially before antireflux surgery.43
TREATMENT
Treatment of PPI refractory GERD should be stepwise with multidisciplinary approach targeting ≥1 of the aforementioned mechanisms.
Lifestyle Modification
Lifestyle modifications such as weight loss; head elevation during sleep; and avoidance of tobacco, alcohol, caffeine, and high fat and spicy foods are frequently prescribed by the treating physicians. It is notable that weight loss and head of bed elevation are the only lifestyle interventions that have been found to be effective for GERD.44
Medical Management
Medical management of refractory GERD is targeted against a specific GERD phenotype established following a thorough examination including a pH-impedance study. The limitations of conventional PPI include short plasma half-life (1–2 hours) and strict adherence to dosing 30–45 minutes before meals. Novel PPI have the potential to largely overcome these limitations. These include stereoisomers with greater bioavailability (esomeprazole and dexlansoprazole), extended release formulations (dexlansoprazole modified release and rabeprazole extended release), long acting PPI with alternate metabolic pathways (tenatoprazole andilaprazole), and potassium competitive acid blockers (revaprazan and soraprazan).
Alginates decrease gastroesophageal reflux by forming a pH neutral raft on the postprandial acid pocket on top of the intragastric food. In a recent multicentre, placebo-controlled, randomised trial, the addition of alginates to PPI produced significant improvement in overall reflux and heartburn scores compared to placebo in patients with persistent symptoms.45
Prokinetics
Prokinetic drugs increase LES pressure, enhance oesophageal clearance of refluxed material, and stimulate gastric emptying rate;46 however, the data on the utility of prokinetics in patients with refractory GERD is not impressive. A meta-analysis found that the addition of prokinetics to PPI had no significant effect on symptom or endoscopic response to GERD but quality of life partially improved.47 In a recent randomised control trial, acotiamide improved symptoms in patients with an overlap of GERD and functional dyspepsia who had persistent symptoms once daily PPI for >8 weeks.48
Reflux Inhibitors
Reflux inhibitors reduce gastroesophageal reflux by inhibiting transient LES relaxations (TLESR). Baclofen, a GABAB agonist, has been shown to decrease acidic and nonacidic reflux episodes and improve symptoms in refractory GERD, both as monotherapy and as an add-on therapy to PPI.49 A recent metanalysis concluded that baclofen reduces the number of reflux events per person, the average length of reflux episodes, and the occurrence of TLESR.50 However, baclofen is often not tolerated well because of neurological side effects. Moreover, there is currently a lack of long-term data on its use in patients with refractory GERD. Lesogaberan and arbaclofen placarbil are peripherally acting GABAB agonists with minimal central actions; however, they are probably less efficacious compared to baclofen.51
Neuromodulators
Tricyclic antidepressants (TCA) and selective serotonin reuptake inhibitors reduce heartburn and oesophageal pain in patients with oesophageal hypersensitivity and functional heartburn. In a randomised trial, only 38.5% of patients in the citalopram group continued to report reflux symptoms compared to 66.7% in the placebo group.52 Similarly, fluoxetine has been shown to reduce the percentage of heartburn-free days compared to the use of omeprazole and placebo in patients with functional heartburn.53 Although TCA reduce visceral hypersensitivity, the results of trials evaluating the use of nortriptyline do not favour their routine use in patients with functional heartburn.54
Surgical Management
Laparoscopic fundoplication or antireflux surgery (LARS) is effective in patients with typical GERD symptoms with acidic or nonacidic reflux. In the long term, LARS provides greater reductions in oesophageal acid exposure after 5 years when compared to PPI therapy;55 however, the results of LARS in refractory GERD are conflicting. In a recent randomised trial, antireflux surgery (laparoscopic Nissen fundoplication) was found to be superior to medical treatment in a highly selected subgroup of patients with truly PPI refractory and reflux related heartburn (67% versus 28%; p=0.007).56 The response to LARS appears to be superior in PPI responders compared to PPI nonresponders (Table 2).55-60 The predictors of favourable outcome following LARS include objective evidence of abnormal oesophageal acid reflux and the presence of typical symptoms of GERD. A recent systematic review evaluated the outcomes of LARS in patients with partial response to PPI. Although heartburn and regurgitation improved immediately after surgery, the symptoms recurred and acid suppressive medication use increased at 10-years follow-up.61 LARS might be considered as a treatment option in patients with refractory GERD with ongoing acid or nonacid reflux, but should be avoided in cases of oesophageal hypersensitivity and functional heartburn. A recent randomised controlled trial of laparoscopic magnetic sphincter augmentation showed promising results in patients with moderate-to-severe regurgitation on once daily PPI. Patients in the laparoscopic magnetic sphincter augmentation group experienced significantly greater improvement in GERD-HRQoL score and resolution of regurgitation compared to those receiving twice daily PPI.62
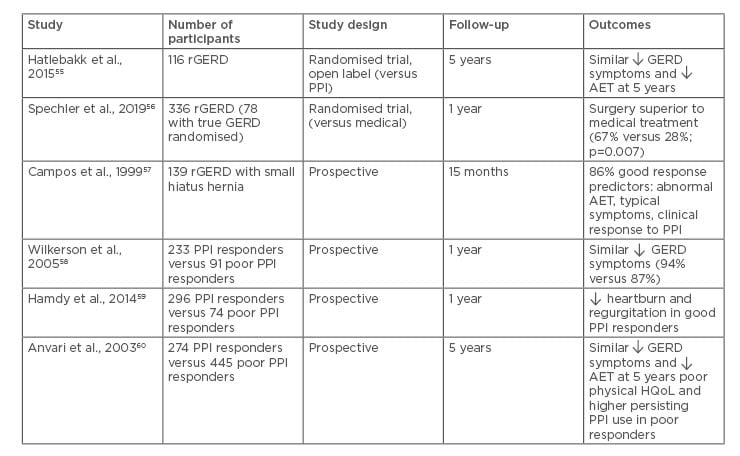
Table 2: Outcomes of antireflux surgery in refractory gastroesophageal reflux disease.
AET: acid exposure time; GERD: gastroesophageal reflux disease; rGERD: refractory gastroesophageal reflux disease; PPI: proton pump inhibitor; HQoL: health related quality of life.
ENDOSCOPIC MANAGEMENT OF GASTROESOPHAGEAL REFLUX DISEASE
Endoscopic management options for GERD include radiofrequency application (the Stretta® procedure [UCI Health, California, USA]), endoscopic fundoplication, and antireflux mucosectomy. In recent years, these treatment modalities have resurfaced because of potential adverse events associated with PPI and antireflux surgery.
Radiofrequency application, or the Stretta procedure, involves delivery of thermal energy to the muscle of gastroesophageal junction and gastric cardia. The mechanism of action is still unclear; however, multiple studies have shown Stretta to be an effective therapy in patients with GERD.63 In a long-term follow-up study including 217 patients, 72% of patients had significantly improved GERD-HRQoL and 64% of patients achieved >50% reduction in the use of PPI after 10 years of follow-up.64
Endoscopic plication devices that are currently available include transoral incisionless fundoplication EsophyX® device (EndoGastric Solutions, Washington, USA), GERDx™ device (G-SURG, Germany), and Medigus Ultrasonic Surgical Endostapler (MUSE™) (MediGus Ltd., Israel).65 Of these, the largest body of evidence is available for transoral incisionless fundoplication using the EsophyX device.66 Whereas, the data is still emerging for the other two plication devices.
The basic principle is similar for endoscopic plication and involves re-enforcement of the gastroesophageal junction using multiple plications or fasteners. In a systematic review and meta-analysis, transoral incisionless fundoplication was found to be safe and effective in patients with refractory GERD. Overall, the adverse event rate was 2%, and PPI therapy could be discontinued in 89% of patients after the therapy.66 Ideal candidates for endoscopic antireflux therapies include those with mild oesophagitis, small hiatal hernia (<2 cm), endoscopic Hill’s Grade II-III, absence of Barrett’s oesophagus, and nonmorbid obesity.65
Besides radiofrequency ablation and endoscopic fundoplication, some of the recent studies have evaluated the efficacy of endoscopic band ligation and cap or multiband assisted antireflux mucosectomy for the management of GERD.66-69 The basic mechanism of these endoscopic techniques is the tightening of gastric cardia as a result of scarring after band ligation or endoscopic mucosal resection. It should be noted that these techniques need to be standardised and evaluated in randomised trials to conclude their efficacy.
CONCLUSION
PPI have revolutionised the treatment of GERD; however, a sizeable proportion of patients are refractory to PPI therapy. Most common aetiologies of refractory GERD include ongoing residual acid reflux, nonacid reflux, oesophageal hypersensitivity, and functional heartburn. Management of refractory GERD should be based on GERD phenotypes after thorough clinical assessment and reflux testing, preferably with combined pH-impedance monitoring (Figure 1). Patient selection for antireflux surgery or endoscopic therapy should be guided by a meticulous examination after excluding oesophageal hypersensitivity and functional heartburn. Treatment of patients with functional heartburn includes proper patient counselling, addressing concomitant psychiatric morbidities and associated functional gastrointestinal disorders, and neuromodulators such as TCA and selective serotonin reuptake inhibitors. Endoscopic antireflux therapies appear to be promising in appropriately selected patients with refractory GERD. Long-term follow-up studies are required before incorporating them into routine clinical practice.
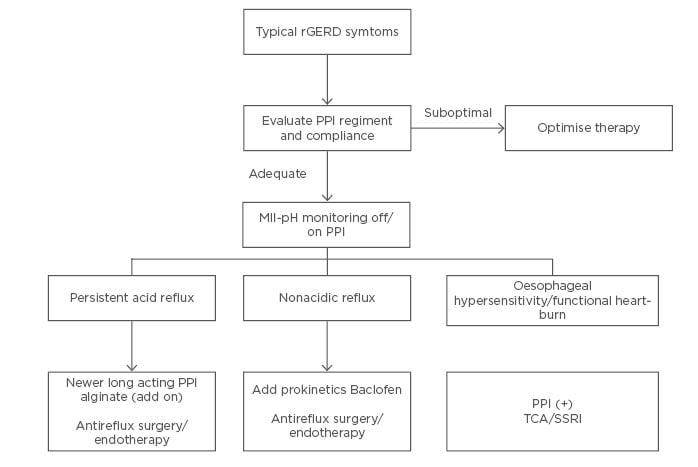
Figure 1: An approach to the management of refractory gastroesophageal reflux disease. MII-pH: multichannel intraluminal impedance-pH; PPI: proton-pump inhibitor; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants.








