Abstract
Inflammatory myofibroblastic tumour (IMT) is a very rare mesenchymal solid tumour commonly found in children and young adults, but also found to be present in older population groups. This case report presents a 33-year-old male patient who was pathologically confirmed to have an IMT of the colon after hemicolectomy and biopsy. The patient presented with abdominal pain and vomiting along with lower gastrointestinal tract bleeding. Colonoscopy of the patient revealed a fungating annular growth in the descending colon. CT also showed annular mass and inflammation of neoplastic process in the entire descending colon and mid-to-transverse colon after hemicolectomy, which may be a result of recurrence of the tumour. Surgical histopathological reports showed infiltrates of polymorphous cells consisting of lymphocytes, eosinophils, and plasma cells.
The aim of this case report was to course through the history, pertinent laboratory test, and plans of management for a case of a young male with an IMT presenting with symptoms of abdominal pain and vomiting.
INTRODUCTION
Inflammatory myofibroblastic tumour (IMT) is a rare mesenchymal solid tumour commonly documented in children and young adults, but also found to be present in older population groups. It is histopathologically characterised by spindle-shaped cells with myofibroblastic proliferation and inflammatory infiltration. Although the common site is primarily in the lung, IMT occurs in many organs including the stomach, small intestine, large intestine, liver, mediastinum, retroperitoneum, and bladder. The clinical presentation of the disease solely depends on the occurrence site of the tumour and the organ involved; however, sometimes the clinical presentation is different with nonspecific features making the diagnosis difficult and challenging. IMT has often been synonymously characterised as a pseudotumour or plasma cell granuloma.
Presented here is an uncommon case of a 33-year-old male Filipino who presented with the primary complaint of abdominal pain and vomiting. He was histopathologically diagnosed with an IMT, suspected through radiological findings to be a result of tumour recurrence.
Learning Objectives
In this case, readers have an opportunity to:
Learn about the history, pertinent laboratory test, and plans of management for a case of a young male with IMT.
Build knowledge of a rare disease with symptoms of abdominal pain and vomiting, and to increase awareness for when dealing with similar kinds of presentation in the future.
Become aware regarding the tumour recurrence even after hemicolectomy, which is the gold standard procedure.
CASE REPORT
A 33 year-old-male presented to the emergency department with symptoms of abdominal pain and vomiting. During the first week of May 2017, the patient started to have abdominal pain on the left lower quadrant (pain scale: 5/10) which was described as nonradiating, sharp in character, and not relieved by position change. There was no associated fever, nausea or vomiting, loose bowel, or constipation, for which consultation had been done in the district hospital. The patient was given some unrecalled medications and was sent home. On May 16th, 2017, the patient had an estimated 2 episodes of haematochezia, approximately 1.0 cup each time. The patient was immediately brought to the emergency room where he passaged 4 episodes of bloody stool, each time approximately 0.5 cup and associated with 1 episode of vomiting containing food particles (nonbilious and nonbloody). The patient was therefore admitted to the hospital.
After the third hospital admission, colonoscopy and biopsy were performed. The colonoscopy revealed a fungating annular growth in the descending colon (approximately 10 cm long), occupying roughly 40% of the colon and displaying ulcerations and small bleeding points. The biopsy showed necrotic tissue with acute and chronic inflammation, and gross findings revealed a few small pieces of tan-coloured tissue with a volume of 1.0 cm3, and all tissues embedded. Microscopic findings revealed small fragments of necrotic tissue and a few fragments of colonic tissue exhibiting normal glands, as well as inflammatory cells in the stroma. On the fifth hospital day, CT of the whole abdomen showed annular mass lesions or inflammatory changes in the distal transverse colon, distal descending colon, and proximal sigmoid colon along with gall bladder polyp (2 mm intraluminal), bilateral nephrolithiasis (1–2 mm), urinary bladder cystitis, and retroperitoneal lymphadenopathies with a normal-sized prostate gland.
The patient then signed a waiver and went home against medical advice on the seventh hospital day (May 25th, 2017) and failed to follow up to the hospital for further planning and management. In June 2017, the patient underwent an exploratory laparotomy with hemicolectomy and the resected part was sent for pathological assessment in another district hospital. The surgical pathological report showed a transmural IMT with irregular defects and positivity for malignant mesenteric lymph nodes. Gross findings showed that the colon section measured 21.1 cm in length and was 8.2 cm wide. Three defects were noted along the entire length measuring up to 2.0 cm and located about 3.1 cm and 8.1 cm from 1 surgical margin. The cut section showed a thickened wall covered by grey white mass measuring about 5 cm in diameter and located near the third and fourth defect. A huge node was noted nearby measuring about 3 cm in diameter. Microscopic findings showed infiltrates of polymorphous cells consisting of lymphocytes, eosinophils, and plasma cells involved transmurally. After the surgical resection, the patient was apparently well and regularly followed up in the same district hospital. In October 2017, the patient started to have recurrences of intermittent fever and abdominal pain again. No medications were taken, and no consultation was given for 2 days. A few hours prior to consultation, the patient presented with haematochezia and anorexia with undocumented weight loss, nausea, and vomiting, and was therefore brought to the emergency department of the hospital. In the emergency room, the patient still had persistent abdominal pain in the left lower quadrant, and had also experienced 4 episodes of haematochezia (about 0.5 cup each time). Despite this, he had no associated vomiting, fever, or difficulty breathing. Consequently, the patient was admitted for further evaluation and management.
The patient did not have any remarkable past medical or family history; however, he was an 18 pack a year smoker and was alcohol-dependent, reporting that he that had drunk bottles of beer 3–4 times a week since his teenage years. The patient worked as an air condition technician. During physical examination, the patient was alert, awake, engaged in conversation, and displayed no signs of cardiorespiratory distress. However, the patient was using a wheelchair for mobility, pale looking, exhibited pale palpebral conjunctiva icteric sclera, had no bipedal oedema, had feeble pulse extremities, and no neck vein engorgement was present. On examination of the chest, there was a symmetrical chest expansion, and no retractions with clear breath sounds. Upon cardiovascular system examination there was a dynamic precordium, normal rate regular rhythm, and no heart murmurs. Abdominal examination showed normoactive bowel sounds; a centrally placed umbilicus; no fullness of flanks; tenderness on palpation of the left lower quadrant; and no palpable spleen, liver, or kidney. Digital rectal examination displayed stool mixed with a reddish tinge of blood on the examining finger, no palpable mass, and no fissures or haemorrhoids. Initial laboratory results showed haemoglobin 70 g/L with thrombocytosis of 564 cm3, total bilirubin 10.26 mg/dL, and direct bilirubin of 8.30 mg/dL.
During the hospital stay, repeated CT was performed showing annular mass, infectious tuberculosis, and inflammation of neoplastic process in the entire descending colon and mid-to-transverse colon (Figure 1). To rule out carcinomatosis along with bilateral nephrolithiasis, the patient was also referred to the surgery department for further evaluation. They were advised for surgery, however the patient was noncompliant.
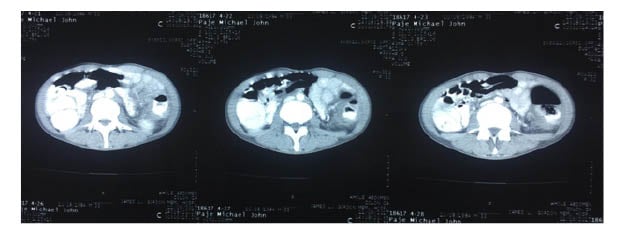
Figure 1: Whole abdominal CT scan with contrast.
This image was taken 4 months after the hemicolectomy when the patient presented with the same symptoms and the presence of an annular mass suggested the recurrence of an IMT of the colon. It shows the neoplastic process in the entire descending colon and mid transverse colon.
IMT: inflammatory myofibroblastic tumour.
DISCUSSION
IMT of the colon is also known as fibrosarcoma, inflammatory pseudotumor, and plasma cell granuloma. This is a rare mesenchymal tumour originally described in the lungs, which is also the most common site among other body parts. Many cases are reported to be found in other parts of the body such as in the mesentery and omentum, stomach, small intestine, large intestine, liver, mediastinum, retroperitoneum, and bladder;1 however, the most common site is the lung, with the most common extrapulmonary sites being the mesentery and omentum.2 An IMT occurs primarily in children and young adults (mean age 10 years), but in more recent years it has been seen that it can occur in any age group and there appears to be no difference in incidence between males and females. It is an aggressive tumour and can occur anywhere in the body.
The cause of an IMT is not yet known, but some believe that its aetiology is multifactorial; this could be as an occurrence following surgery or trauma, or infection with Epstein–Barr or human herpes viruses attributable to reactive inflammatory mediators, mainly cytokines. New studies suggest that it is also associated with gene mutation. In approximately 50% of the cases an ALK gene mutation has been reported. A chromosomal rearrangement in 2p23, the site of ALK gene, is present in a subset of these tumours.2 Some studies have shown that this chromosomal abnormality may be attributable to a clonal origin, and is not necessarily a reactive process; therefore, an IMT should be considered as a true neoplasm.
Many cases of IMT of the colon have been reported, some with recurrence either after surgery or chemotherapy. The patients present with common gastrointestinal symptoms similar to other common disease conditions such as abdominal pain, vomiting, melena, or haematochezia, and sometimes with positive faecal occult blood and features of malignancy such a weight loss and night sweats. Patients can also present with unknown cases of anaemia, fever, and intussusception, mimicking appendicitis and bowel obstruction. The location of the lesion is mainly found in the sigmoid colon, mid-transverse colon, ileum ascending colon, appendix, left colon, and the proximal descending colon.
Differential diagnosis of IMT includes malignancy, submucosal tumour, lymphoma, solitary fibrous tumour, and fibromatoses or desmoid tumour. The diagnosis of an IMT includes complete evaluation of family medical history along with thorough physical examination. The diagnostic tools also include screening colonoscopy and lower gastrointestinal series;3 however, examination of biopsy specimen under microscopy is the gold standard for arriving at a conclusive diagnosis. Inflammatory fibroid polyp, fibromatoses (desmoid tumour), gastrointestinal stromal tumours, leiomyoma, leiomyosarcoma, and schwannoma have similar pathological findings with IMT.
Inflammatory fibroid polyp is typically submucosal and consists of a mixture of small granulation tissue-like vessels, spindle cells, and inflammatory cells, mainly eosinophils. Fibromatoses are composed of spindled or stellate cells, arranged in parallel with evenly spaced blood vessels and a collagenous background. Immunohistochemistry also gives valuable information regarding the tumours such as gastrointestinal stromal tumour stains for CD34 and CD117 (c-kit).1 Leiomyoma and leiomyosarcoma stain positively for desmin and actin, and negatively for CD117 and CD34. IMT stains smooth muscle actin and vimentin for >90%, for desmin approximately 10–70%, for keratin 30–77%, and ALK 35–60%. However, ALK positivity is not specific for IMT because it also stains with CD68 and CD34 to variable degrees. In general, immunohistochemistry does not play a major role in confirming a diagnosis due to variable expression and lack of specificity of myofibroblastic markers. ALK positivity is helpful if present but its absence does not exclude the diagnosis of IMT.2
Chemotherapy, radiation treatment, nonsteroidal anti-inflammatory drugs, and steroids have been used as treatment modalities, but surgical resection is considered as the treatment of choice. Chemotherapy using cyclosporine A, crizotinib in ALK rearrangement, and infliximab have shown to be successful.3 Chemotherapy may also help if the condition recurs, if there is local invasion, or a distant metastasis presents, yet chemotherapy is generally administered following surgery. There are no reports solely on radiotherapy treatment, but researches have shown that radiation can shrink the tumour prior to surgery for easy removal. In young children, if the tumour cannot be surgically removed, then corticosteroid administration can be beneficial. Orally administered nonsteroidal anti-inflammatory drugs may be effective as anti-inflammatory medication along with other treatment tools. Occasionally some tumours are known to disappear over time without any treatment.4
An early diagnosis and prompt treatment of IMT of the colon generally yields a better outcome than a late diagnosis and delayed treatment. The prognosis on timely surgical removal of the tumour is generally good. Some tumours are known to spontaneously regress and disappear but upon complete excision and removal they are generally not known to recur; in some instances, however, they can recur and even metastasise. It is unclear what kind of factor is associated with prognosis,3 and it is unclear what the best way of treatment of recurrence or metastasis is during long-term follow-up. It is important to follow-up on patients diagnosed with an IMT, even if they have undergone surgical resection.
CONCLUSION
The clinical presentation of the disease solely depends on the site of occurrence of the tumour and organ involved and the variability in presentation. Most of the cases are benign; however, these tumours also have malignant potential and some even metastasise. Therefore, being complacent with one diagnosis, typically with nonspecific features, can predispose to misdiagnoses. Complete surgical excision is the mainstay of treatment and the prognosis is generally good, with only rare reports of malignant transformation, recurrence, or distant metastases. Follow-up with long-term clinical and radiological review is advised to ensure early detection of recurrence or metastases.








