BACKGROUND AND AIMS
Patients with inflammatory bowel disease (IBD) are frequently on immunosuppressive treatments that increase the risks of infection. To date, there are limited data on the disease course of coronavirus disease (COVID-19) in patients with IBD, including the impact of clinical characteristics and medications. The authors utilised the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD), an international registry of patients with IBD who have had COVID-19, to evaluate the association of demographics, clinical characteristics, and immunosuppressant treatments on COVID-19 outcomes. This work was an updated analysis of SECURE-IBD following the first publication from this database.1
MATERIALS AND METHODS
Age-standardised mortality ratios were calculated using reference populations from China, Italy, and the USA.2-4 The primary outcome was severe COVID-19, defined as a composite of intensive care unit admission, ventilator use, and/or death. Multivariable logistic regression was used to understand the independent impact of variables on severe COVID-19.
RESULTS
A total of 959 cases from 40 countries were reported (median age: 43 years; 52% male). A total of 86 patients (9%) had severe COVID-19, 320 (33%) were hospitalised, and 37 patients died (3.9% case fatality rate). Age-standardised mortality ratio for patients with IBD were 2.0 (95% confidence interval [CI]: 1.4–2.7), 1.7 (95% CI: 1.1–2.2), and 1.9 (95% CI: 1.3–2.5), relative to data from China, Italy, and the USA, respectively. On multivariable analysis, risk factors for severe COVID-19 among patients with IBD included increasing age, ≥1 comorbidities in addition to IBD, systemic corticosteroids, and sulfasalazine or 5-aminosalicylate use (Table 1). TNF antagonist treatment was not associated with severe COVID-19 (adjusted odds ratio: 0.9; 95% CI: 0.5–1.8).
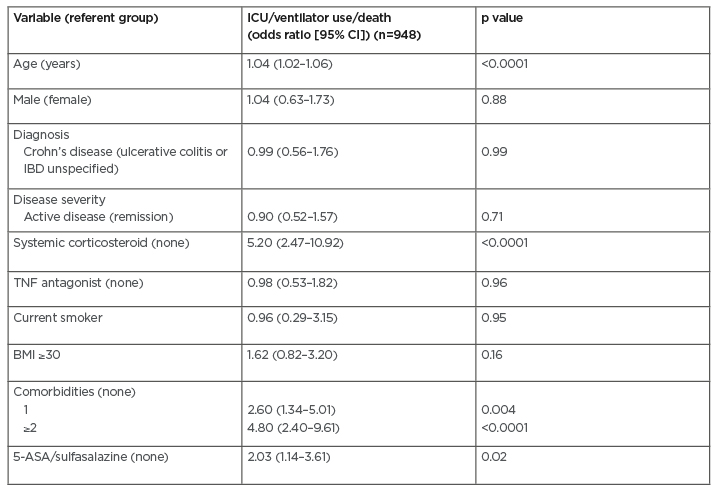
Table 1: Multivariable analysis of risk factors for severe coronavirus disease (COVID-19).
5-ASA: 5-aminosalicylic acid; CI: confidence interval; IBD: inflammatory bowel disease; ICU: intensive care unit.
CONCLUSIONS
Strengths of the study included a large, international population of patients with IBD. Limitations included the fact that this was a convenience sample, with potential for reporting bias. The clinical implication of these findings is that patients with IBD who are older with multiple comorbidities, and those on systemic corticosteroids, are at higher risk of severe COVID-19. In contrast, TNF antagonists do not appear to increase the risk of poor outcomes, and these data provide reassurance that patients should continue these medications. Future research is needed to better understand the impact of other IBD medications, including different classes of biologics.








