Interview Summary
Chronic obstructive pulmonary disease (COPD) is a condition for which there is a portfolio of devices that can be used for treatment. These include pressurised metered dose inhalers (pMDI), pMDIs with a spacer, soft mist inhalers (SMI), dry powder inhalers (DPI), and nebulisers. In this interview with EMJ, Omar Usmani, Professor of Respiratory Medicine at the National Heart and Lung Institute (NHLI), Imperial College London, UK, summarised how nebuliser therapy works, the reasons for using these devices, and the characteristics of patients who typically benefit. The importance of peak inspiratory flow (PIF) was discussed, and how suboptimal PIF (sPIF) should be managed. Usmani explained the concept of nebuliser efficiency and how it can be objectively measured, illustrating that not all nebulisers are the same. A case study was described, showing the need to assess inhaler technique, and the benefit of switching to nebuliser therapy when the patient demonstrates poor engagement with inhaler devices. Usmani concluded by emphasising that nebulisers have a crucial role to play in the everyday management of patients with COPD.INTRODUCTION
Nebulisers are devices used to rapidly deliver high doses of medication to the lungs in people with respiratory disease. They convert liquid medication into an aerosol that is then inhaled through a facemask or mouthpiece. Nebulisers provide a rapid onset of action of the administered drug, proven efficacy, a favourable safety profile, and ease of use.1 They are suitable for patients of all ages, with various underlying respiratory diseases, and for those with cognitive disorders.1,2 Physicians can influence efficacy by choosing an efficient nebuliser which maximises the amount of medication reaching the lungs.
A growing body of evidence supports using nebulisers for maintenance therapy of respiratory disease in the outpatient setting.3
The management of patients with COPD can involve a range of inhalation devices, including pMDIs, DPIs, SMIs, and nebulisers.4 While pMDIs, DPIs, and SMIs are appropriate for many patients, they may not be the optimal delivery method in certain patients, such as those with sPIF and impaired physical and/or cognitive capabilities.4 Nebulised treatment may be a beneficial alternative in these populations.4,5 For example, nebuliser therapy may be preferred in patients with COPD, who are unable to effectively use pMDIs or DPIs despite instruction and training ( Table 1).3
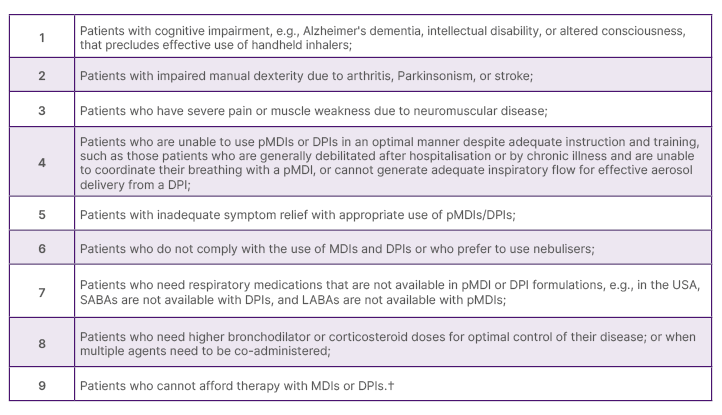
Table 1: Clinical scenarios where maintenance nebuliser therapy may be preferred in patients with chronic
obstructive pulmonary disease.*3
*Based on the consensus of the authors’ expert opinion and clinical experiences.
†For Medicare-eligible patients.
DPI: dry powder inhaler; LABA: long-acting β agonists; pMDI: pressurised metered dose inhaler;
SABA: short-acting β agonists.
This can include patients who are unable to coordinate their breathing with a pMDI, or who have sPIF, as discussed above, and cannot produce sufficient inspiratory flow for effective aerosol delivery from a DPI.3 Nebulisers avoid the need for manual dexterity and complex hand–breath coordination.4 Potential disadvantages that have been reported for some jet nebuliser devices include the many parts that need to be cleaned and maintained, as well as a treatment time which is typically relatively long.6 Jet nebulisers are also heavy and bulky (compressor/tubes/nebuliser), and loud when operating.6 Their performance furthermore depends on compressor/nebuliser pairing, with a large variability in dose delivery depending on their combination, and a residual volume is to be expected.6
SUBOPTIMAL PEAK INSPIRATORY FLOW
Usmani discussed the importance of PIF, its prevalence in patients with COPD, and how patients with sPIF should be managed.
What is Peak Inspiratory Flow and Why is it Important?
PIF refers to the maximal airflow obtained during an inspiratory manoeuvre.5 The delivery of drugs via DPIs depends on PIF, since they require an initial fast inhalation.7,8 The optimal PIF for DPIs is generally ≥60 L/min or ≥30 L/min, although this depends on the type of device.5 Studies have shown that sPIF is associated with worse COPD-related symptom burden, and a greater likelihood of COPD-related hospital readmissions.5,7
How Common is Suboptimal Peak Inspiratory Flow in Patients with Chronic Obstructive Pulmonary Disease?
A study by Mahler et al.9 found that the overall prevalence of sPIF was 44.6% in patients with COPD, who were hospitalised for any cause, and were using at least one DPI at admission.9 In this study, sPIF was defined as <60 and <30 L/min for low-to-medium high-resistance DPIs and high-resistance DPIs, respectively.9
How are Patients with Suboptimal Peak Inspiratory Flow Generally Managed?
In the UK, many patients with severe and very severe COPD and sPIF are still managed with DPIs. However, DPIs require an initial fast inhalation to break down the medicine correctly, so that it reaches far enough down into the lungs.7 Patients with sPIF can therefore struggle with DPIs.
How Should Patients with Suboptimal Peak Inspiratory Flow who Struggle to Inhale Sufficient Medicine with Dry Powder Inhalers Be Managed?
Studies have shown that patients with sPIF do respond to nebulised therapy.5,7 For example, a study by Loh et al.7 demonstrated that in patients with sPIF, all-cause and COPD 30- and 90-day readmission rates were significantly lower for those discharged with nebuliser compared with DPI therapy.7
THE ROLE OF EFFICIENCY IN NEBULISER THERAPY: NOT ALL NEBULISERS ARE THE SAME
Usmani outlined the UK recommendations on nebuliser efficiency, its relevance to patients, and how efficiency can be objectively measured.
What Do UK National Institute for Health and Care Excellence Guidelines Say?
The UK National Institute for Health and Care Excellence (NICE) guidelines recommend using a nebuliser system that is known to be efficient.10
How Important is it That Patients Have Access to Efficient Nebulisers?
Mistakes can occur when using inhaler devices, for example, due to poor hand–breath coordination.11 A meta-analysis of 10 studies including 1,360 patients found that more than three in four adults with COPD used pMDIs incorrectly.11 Patient errors in using pMDIs or DPIs can lead to a poorly-controlled disease status.12 Nebulisers are an effective alternative to inhalers, and can be prescribed for patients with COPD who are unwilling or unable to use inhaler devices.12 Efficiency is a key factor when selecting a nebuliser system. An efficient nebuliser rapidly delivers a high amount of medication to the lungs, which can result in a positive effect on patient adherence.13
How Can Nebuliser Efficiency be Objectively Measured?
Nebuliser efficiency can be objectively measured using the respirable drug delivery rate (RDDR), which is calculated as the product of respirable fraction (representing the proportion of particles <5 μm) and aerosol output rate, defined by the amount of salbutamol deposited on the inspiratory filter within the first minute of nebulisation. Research by Fischer et al.13 has demonstrated major differences in the efficiency of nebuliser systems.13 The study evaluated 15 types of jet nebuliser systems, which were filled with 2 mL of 0.1% (w/v) salbutamol.13 The nebuliser system with the highest RDDR had a value that was approximately three-fold higher than the system with the lowest RDDR.13 PARI devices demonstrated superior performance over other systems (Figure 1).
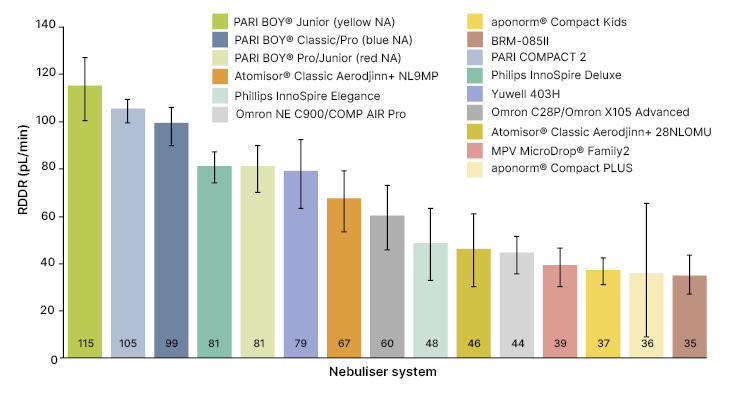
Figure 1: Mean respirable drug delivery rate across 15 jet nebuliser types.13
Error bars indicate 95% confidence interval.
Reproduced with permission from PARI GmbH.
aponorm® Compact Kids: Microlife, Widnau, Switzerland; aponorm® Compact PLUS: Microlife; Atomisor® Classic Aerodjinn+ 28NL0MU: DTF Medical, Saint-Etienne, France; Atomisor® Classic Aerodjinn+ NL9MP: DTF Medical;
BRM-085II: Bairui Medicine Co, Guangzhou, China; MVP MicroDrop®
Advanced: OMRON Healthcare, Kyoto, Japan; Omron NE C900/COMP AIR Pro: OMRON Healthcare; PARI BOY® Classic/Pro (blue NA): PARI GmbH; PARI BOY® Junior (yellow NA): PARI GmbH; PARI BOY® Pro/Junior (red NA): PARI GmbH; PARI COMPACT2: PARI GmbH; Philips InnoSpire Deluxe: Philips, Amsterdam, the Netherlands; Philips InnoSpire Elegance: Philips; Yuwell 403H: Yuwell, Shanghai, China.
NA: nozzle attachment; RDDR: respirable drug delivery rate.
CASE STUDY
Usmani discussed the case of a patient in the UK who had chronic severe COPD, and was stabilised after using an efficient nebuliser.
While being treated at a previous healthcare centre, the patient had described increasing breathlessness over the last 3–6 months.
The patient had been tried on different inhalers during that period, starting with a pMDI and a spacer, followed by an SMI, and then a DPI with a long-acting β2 agonist, and a long-acting muscarinic antagonist as a combination therapy. The patient had attended due to the lack of clinical benefit. The healthcare professionals involved in the case had not assessed the patient’s ability to engage with the inhaler device. Instead, they had assumed that the patient was using the device correctly, but the drugs were ineffective. Dosages of the long-acting β2 agonist, or the long-acting muscarinic antagonist combination were therefore increased, but with no improvement in efficacy.
At that point, the patient was referred to Usmani’s clinic, where their inhaler technique was assessed. This revealed that the patient had early-onset dementia and poor coordination, which meant they were unable to correctly use a pMDI with a spacer, an SMI, or a DPI. They also had sPIF, which was inhibiting their use of a DPI.
Usmani emphasised that when a patient is unresponsive to treatment, healthcare professionals need to assess their engagement with the inhaler to confirm that they are using the device properly, rather than assuming that the medicine is incorrect, or that the patient is non-adherent or non-compliant.
The patient was switched to an efficient nebuliser with ipratropium and salbutamol, a short-acting muscarinic antagonist, and a short-acting β2 agonist. The prescription was to use the device up to four times a day, an increase from their inhaler prescriptions of once or twice daily. However, it was found that the patient received sufficient drugs to obtain clinical benefit by using the nebuliser just twice a day. In addition to alleviating their breathlessness, nebuliser therapy led to improved abilities to perform activities of daily living. The patient was able to walk around the house longer than previously, and undertake more housework than before, all leading to an overall improvement in quality of life.
Usmani stressed that nebulisers are part of the armamentarium of devices that can be used in the everyday management of patients with COPD, not just in acute situations, with the full portfolio also including pMDIs, pMDIs with a spacer, DPIs, and SMIs.
FUTURE DIRECTIONS AND CONCLUSIONS
Usmani provided his perspectives on the future of nebuliser therapy. and his take-home messages on the use of this therapy in the everyday management of patients with COPD.
What is Your Opinion on the Development and Future Availability of Additional Medications for Nebuliser Treatment of Patients with Chronic Obstructive Pulmonary Disease?
It is an exciting time for nebuliser therapy, with the development of novel drugs and devices specifically for patients with COPD. Efficient nebulisers have become more portable, with handheld devices that can be battery operated, making them more convenient for the patient to use. Coupled with that, work is underway to deliver inhaled α1 antitrypsin to patients with COPD. α1 deficiency is a genetic disorder that predisposes to COPD, and currently, treatments are administered intravenously. In addition, a phosphodiesterase ¾ combination is currently under the U.S. Food and Drug Administration (FDA) review for approval in the USA, to be given via the nebulised route for patients with COPD. Standalone PDE3 and PDE4 nebulised drugs are also in development.
What is Your Take-Home Message Regarding the Role of Nebuliser Therapy in the Management of Patients with Chronic Obstructive Pulmonary Disease?
As shown in the case, nebulisers are part of the portfolio of devices that can be used in the everyday management of patients with COPD.
The inhalation technique is really important, and nebulisers play a role in helping patients with COPD achieve clinical benefit for the very reason that they may not be able to use other devices.
Another key point is that, often, patients do not realise that they are using a device incorrectly. Instead, they attribute the lack of clinical benefit to ineffective medication. Engaging with patients, and observing how they use a device, will show whether or not there are errors. If the patient is still unable to use a device properly after training, then another device, such as a nebuliser, should be considered.
When choosing a nebuliser, it is important to note that not all devices are the same. An efficient nebuliser will ensure that patients receive a sufficient quantity of medication in the lungs, and make it more likely that they will adhere to the treatment.







