Abstract
Post-endoscopic retrograde cholangiopancreatography (ERCP) air leak (PEAL) syndrome is a rare complication that includes pneumothorax, pneumomediastinum, pneumoperitoneum, air embolism, and subcutaneous emphysema. A 71-year-old female diagnosed with mild acute biliary pancreatitis, who underwent ERCP for stone retrieval developed neck, chest, and abdominal pain, as well as swelling of the neck, along with crepitus all along the neck and face. CT scan showed pneumoperitoneum, pneumomediastinum, and subcutaneous emphysema. The patient was diagnosed as a case of PEAL syndrome, and was managed conservatively. She ultimately underwent an uneventful cholecystectomy with peroperative stone retrieval. PEAL syndrome, albeit rare, can be a potentially life-threatening complication following ERCP, which requires continuous monitoring. It may be managed conservatively, endoscopically, or surgically.
Key Points
1. Post-endoscopic retrograde cholangiopancreatography (ERCP) air leak (PEAL) syndrome is any of the various combinations of pneumothorax, pneumomediastinum, pneumoperitoneum, air embolism, and subcutaneous emphysema occurring secondary to injury during ERCP.
2. Although rare, it is one of the life-threatening complications of ERCP that is often overlooked and misunderstood.
3. Management of post-ERCP air leak syndrome is mainly based on conservation treatment; however, a select few require surgical intervention for correction.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) includes specialised techniques, such as sphincterotomy, balloon dilation, nasobiliary drainage, and stent placement. This makes it more challenging than oesophagogastroduodenoscopy, and gives rise to a unique set of complications, such as pancreatitis, cholangitis, gastrointestinal bleeding, and perforation.1 The rarest of these complications is perforation, with an incidence of 0.6–0.8%, which can lead to conditions ranging from post-ERCP air leak (PEAL) syndrome to peritonitis.1,2
PEAL syndrome encompasses a range of clinical manifestations attributed to iatrogenic perforation of the duodenum, ampulla, or biliary tree after ERCP, potentially resulting in pneumoperitoneum, pneumomediastinum, pneumothorax, air embolism, or subcutaneous emphysema.1-3 It is important to note that pneumobilia is not considered a part of PEAL syndrome.4 In this context, the authors present a case of PEAL syndrome, which, to their knowledge, is the first case report of its kind from Pakistan. The study introduces the term of PEAL syndrome, and outlines recommendations, along with managerial principles for the treatment of this complication. Informed written consent was taken from the patient regarding the use of images and publication of the case.
CASE DESCRIPTION
A 71-year-old female of South Asian origin presented to the surgical outpatient department with an acute history of abdominal pain associated with nausea and vomiting for 3 days. She had moderately severe, dull, intermittent epigastric pain radiating to the back. Her past medical history was unremarkable, and she did not have any relevant history of drug use or genetic disorders.
On examination, she was vitally stable, with a pulse rate of 77 bpm, blood pressure of 110/75 mmHg, respiratory rate of 15 breaths per minute, temperature of 98 °F, and SpO2 of 99% at room air. She was thin and lean, and her systemic examination was unremarkable. An abdominal ultrasound showed a well-distended gallbladder with multiple echogenic calculi (the largest measuring 0.8 cm). Incidentally, two echogenic calculi (the largest measuring 1.1 cm) were seen in the common bile duct, causing proximal dilation to 1.3 cm. Her serum amylase was significantly elevated at 756 U/L. The rest of her investigations were within normal limits, and her Ranson’s score was calculated to be less than three.
She was diagnosed with acute biliary pancreatitis of the mild category, and was managed conservatively with adjusted doses of ketorolac, ondansetron, and omeprazole. An ERCP was planned by the Gastroenterology team for stone retrieval. During ERCP, cannulation of the major papilla was attempted with the standard guide-wire technique. However, it could not be done successfully, even after multiple attempts. The head endoscopist took the decision to proceed with a needle knife papillotomy to gain access; however, cannulation could still not be done successfully, and the procedure was abandoned. The Gastroenterology team rescheduled the ERCP procedure to try again after a certain time.
The patient had an uneventful initial recovery; however, on the first post-procedure day, she reported pain in her neck, chest, and abdomen, along with lethargy. On examination, she had gross distension of the neck with the presence of crepitus all around the neck and on both cheeks. Her chest X-ray showed pneumomediastinum and pneumoperitoneum, as shown in Figure 1.
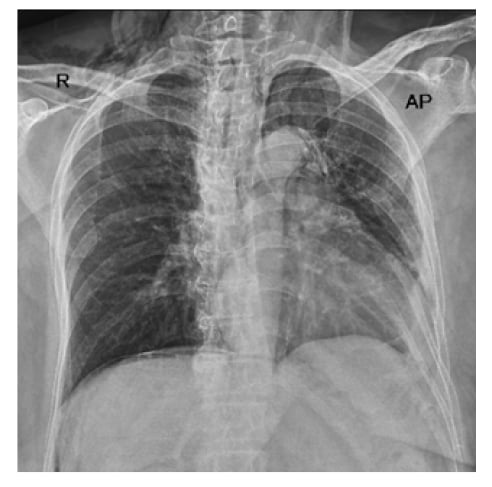
Figure 1: Chest X-ray showing pneumomediastinum and pneumoperitoneum.
She underwent an urgent CT scan of the chest and abdomen under controlled conditions. It showed subcutaneous emphysema, pneumomediastinum, and pneumoperitoneum with no obvious site of perforation, as shown in Figure 2. The patient was shifted to the High Dependency Unit, and was managed conservatively with continuous monitoring and symptomatic treatment. She was started on an adjusted dose of cefuroxime (750 mg IV every 8 hours for 7 days). She was initially kept nil per os, and a nasogastric tube was kept in place. With no progressive clinical deterioration, the nasogastric tube was removed and she was allowed to ingest liquids and then semi-solids orally after 48 hours. She received maintenance fluids during this period; however, she was not started on total parenteral nutrition. By the fourth post-procedure day, she was allowed a soft diet.
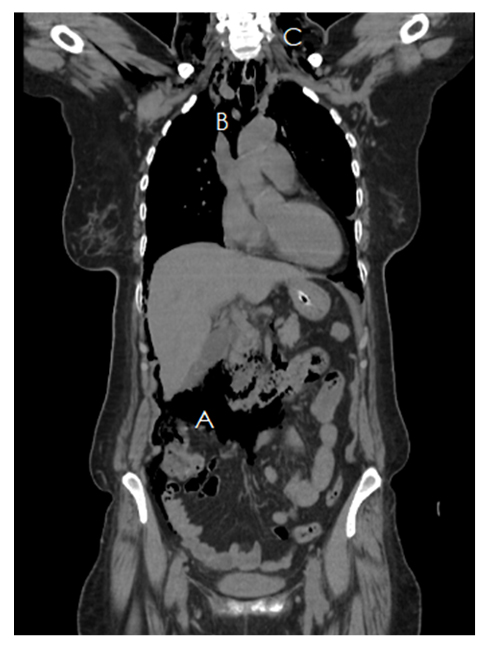
Figure 2: CT Scan showing pneumomediastinum (A), pneumoperitoneum (B), and subcutaneous emphysema (C).
By the seventh post-procedure day, she had complete resolution of symptoms, and her amylase spontaneously normalised. On account of her improvement, her rescheduled ERCP was called off, and she was discharged with regular follow-ups. She was readmitted 6 weeks later and underwent an uneventful cholecystectomy with stone retrieval from the biliary ducts.
DISCUSSION
During ERCP, perforations may occur in the duodenum via stents; in the ampulla via sphincterotomy; or in the biliary channels via guidewires, stents, and balloons. Mortality rates have been reported to be as high as 34.4% (with an average of 0.3–3.5%).1,2 Multiple risk factors have been identified, including patient factors, such as senility, female sex, and hypertension; disease factors, such as sphincter of Oddi dysfunction, juxtapapillary duodenal diverticula, biliary strictures, papillary stenosis, Billroth II reconstruction, and pre-cut sphincterotomy; and technical factors, such as difficult cannulation, an unskilled endoscopist, and prolonged procedure time.5,6
It is postulated that there are two major mechanisms as to how the air traverses during PEAL syndrome. The first is that air enters the retroperitoneal space after an interruption in the duodenal barrier, tracking the deep fascial places from the retroperitoneal space to peritoneum, mediastinum, and subcutaneous tissue, which are contiguous with the diaphragmatic hiatus and major thoracic airways. The second states that air passes to the right pararenal spaces, from where it reaches the diaphragmatic hiatus, leading to pneumomediastinum, pneumothorax, and cervical subcutaneous emphysema.7
A widely used classification system for ERCP-related perforations is the Stapfer classification, as shown in Table 1.7,8 However, it is not commonly used in clinical practice, due to its focus on the location and severity of perforation. For this reason, alternative classifications have been proposed that take into account the instrument causing the perforation.2
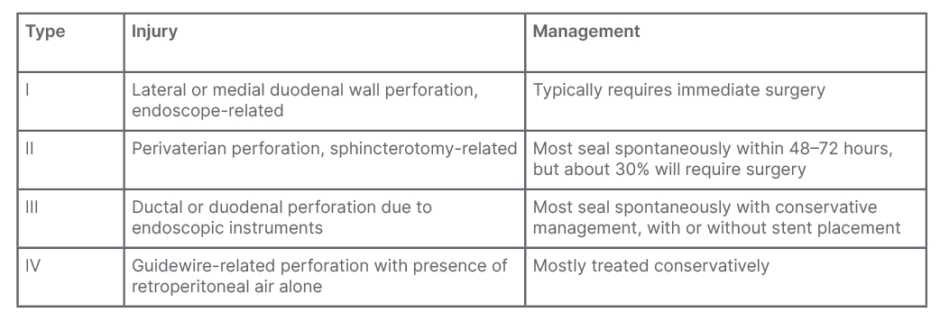
Table 1: Srapfer et al.7 classification of iatrogenic perforation during endoscopic retrograde cholangiopancreatography.
The European Society of Gastrointestinal Endoscopy (ESGE) reports causes of post-ERCP perforation as endoscopic sphincterotomy (56%), guidewire manipulation (23%), stricture dilation (4%), and stent insertion/migration (3%).9
Clinical manifestations of ERCP-related perforations include epigastric or generalised abdominal pain associated with non-specific complaints, like nausea, vomiting, and dyspepsia. Depending on the degree of perforation, the subsequent development of PEAL syndrome may have additional features.5,6
The ESGE recommends an early contrast study during ERCP or CT scan, to assess for perforation in high degree of suspicion.4,9
Treatment of the perforation itself includes conservative, endoscopic, and surgical options. Conservative treatment involves parenteral nutrition (for malnourished individuals, if feeding is not resumed in 7 days), antibiotics, nasogastric/nasoduodenal drainage, and symptomatic relief.9 Endoscopic options include using endoscopic clips alone for perforations <1 cm, or combined endoclips and endoloops, combined endoclips and double endoscopic band ligations, and endoscopic device closures for perforations >1 cm. Other options include vacuum therapy, tulip-bundle, metallic stenting, purse-string sutures, and fibrin glue.2,5,6 The most commonly employed tool, however, is to divert fluids from the perforation site via stenting.9
Surgery is required in approximately one-quarter of the cases. Surgical options typically include Graham patch repair or primary repair, and are offered to patients developing chemical peritonitis, purulent peritonitis, larger peritoneal collections, major contrast medium leak, unresolved problems like retained hardware that cannot be solved non-surgically, or if the condition does not improve with conservative/endoscopic management.7,9 Once indicated, early surgery is preferred over delayed surgery due to increase in morbidity.6
The ESGE recommendations also mention the implementation of a written policy regarding management of iatrogenic perforations, reporting endoscopically identified perforation and early utilisation of CT scan in high index of suspicion.9
PEAL syndrome comprises five components, each with its own set of management principles: pneumothorax, pneumomediastinum, pneumoperitoneum, air embolism, and subcutaneous emphysema.4,6
Development of pneumothorax after ERCP may present with tachypnoea, respiratory distress, tracheal deviation, and hypoxia.10 It is typically on the right side, but may be bilateral.7 Diagnosis can be made clinically or through a chest X-ray or CT scan of the chest. Usual management involves supportive care of the airway, breathing, and circulation with continuous monitoring. Patients are placed in a position where they are comfortable, typically reverse Trendelenburg. In cases of tension pneumothorax or clinical deterioration, decompression via an intercostal drain is indicated.10
Post-ERCP pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema typically do not require treatment, since the air is gradually absorbed. However, hyperbaric O2 therapy may expedite the process.8,11 Periocular subcutaneous oedema requires close monitoring for pressure effects on the orbit manifested by proptosis, raised intraocular pressure, or worsening of vision. In any of these symptoms, a decision may be warranted to perform emergency orbital decompression via lateral canthotomy/cantholysis, needle decompression, or by decompression.11
Air embolism is an even rarer component of PEAL syndrome that can be fatal, and should be considered in patients with sudden clinical deterioration during, or immediately after, the procedure. Diagnosis may involve the use of precordial Doppler ultrasound and transoesophageal echocardiogram. Treatment mainly consists of supportive measures, including airway, breathing, and circulation management. Fluid resuscitation and vasopressors have been shown to improve cardiac output, while hyperbaric O2 therapy is initiated in suspected cerebral air embolism.3 A detailed algorithm for management is displayed in Figure 3.
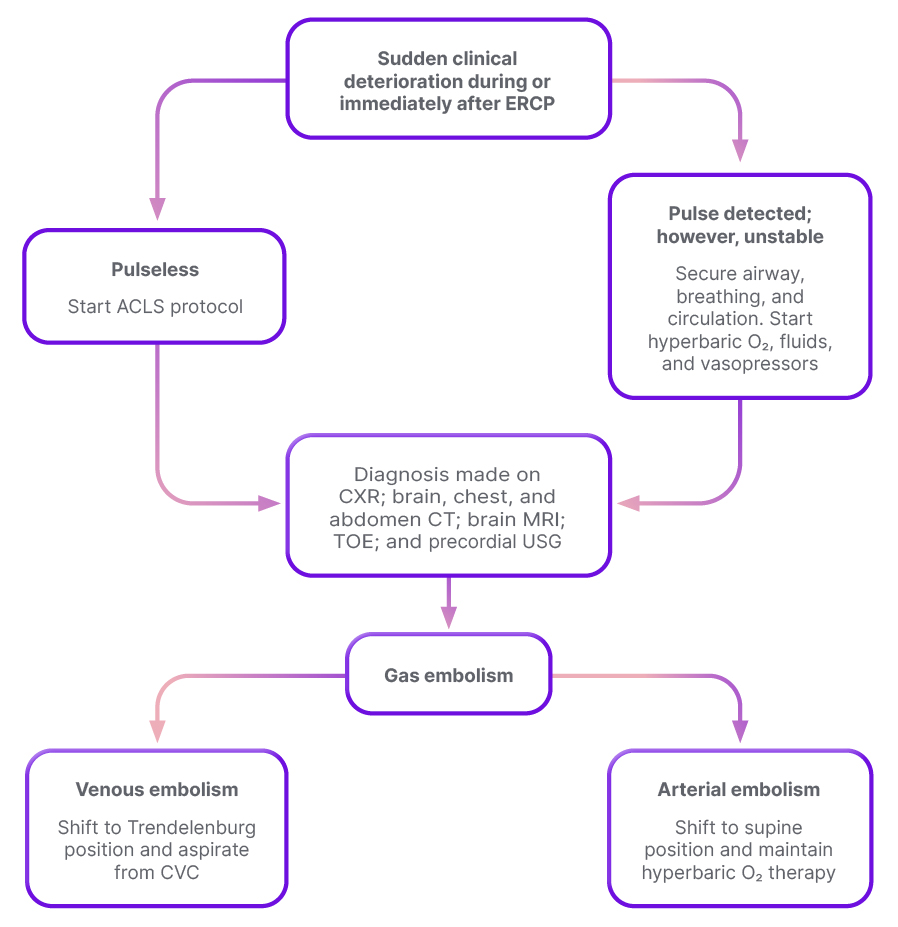
Figure 3: Algorithm for management of post-endoscopic retrograde cholangiopancreatography air embolism.
Adapted from Lanke et al.3
ACLS: advanced cardiovascular life support; CVC: central venous catheter; CXR: chest X-ray; ERCP: endoscopic retrograde cholangiopancreatography; TOE: transoesophageal echocardiogram; USG: ultrasonography.
PATIENT’S PERSPECTIVE
The patient reported that she initially felt very traumatised by the whole scenario, as her whole face, neck, chest, and the rest of her body were swollen. She further said she thought she could never look at herself again, but the doctors reassured her that everything was in control, and they were right. Slowly, things got better and she went back to the way she was.
CONCLUSIONS
PEAL syndrome is a rare complication of ERCP that is potentially life-threatening. Most components of PEAL syndrome are managed via supportive care, with most patients managed conservatively. However, a subset of patients will require endoscopic or surgical interventions to treat the underlying perforation.







