Abstract
Cancer survivors face a range of physical symptoms, emotional and practical difficulties, and late and long-term effects of treatment. Follow-up care aims to monitor the effectiveness and safety of treatments, and detect recurrence or metastasis. However, survivorship care extends beyond clinical aspects, and should include all forms of necessary support, such as psychological, informational, and practical assistance. The European Cancer Patient’s Bill of Rights emphasises patient-centred care, and the right to access information, receive personalised care, participate in decision-making, and receive psychological and social support. There are national plans for cancer care in place in Greece, and it is essential to acknowledge patient preferences and the acceptability of emerging plans for the ever-expanding population of survivors of cancer.
A discrete choice experiment (DCE) will be conducted to identify and quantify the preferences of Greek patients with cancer towards a care model that reflects the current state of cancer care, while also providing valuable insights into what patients consider important. By understanding patients’ preferences, the study aims to identify areas for improvement, and contribute to the development of more patient-centred care models in Greece. This study will mark the first systematic measurement and quantification of preferences of patients with cancer in the Greek context.
This paper presents the development of the qualitative phase of the DCE, which focused on identifying the characteristics that are both important and relevant to patients with cancer. Further, it outlines the protocol for the subsequent stages of the study, which involve the DCE experimental design, the data collection, and analysis and dissemination of the findings.
Key Points
1. This study will explore cancer survivor preferences in Greece, informing patient-centred care models and policy decisions for improving post-treatment support.2. A discrete choice experiment is in development to identify preferences of patients with cancer for follow-up care models, offering insights important for enhancing survivorship care in Greece.
3. Health policymakers and clinicians must recognise the importance of patient preferences in survivorship care. Tailoring follow-up models to align with patient needs and preferences can optimise outcomes and promote patient-centred cancer care.
INTRODUCTION
After being diagnosed with cancer, patients embark on a journey that involves ongoing treatments, and regular visits to healthcare providers. During the initial treatment, and following its completion, the main focus of follow-up care is to monitor the effectiveness and safety of the treatments received, and to detect any recurrence or metastasis of the disease.1 Survivors of cancer, whether considered disease-free or not, often experience late and long-term effects of treatment, a range of physical symptoms, and emotional and practical difficulties.2 Hence, it is widely recognised that care models for survivors of cancer should extend beyond the clinical aspects of care, and include all forms of necessary support, such as psychological, informational, and practical assistance to patients, with a focus on their overall experience and outcomes.3
According to the European Society for Medical Oncology (ESMO)’s patient guide on survivorship,4 survivorship care encompasses the health and physical, psychological, social, and economic issues that affect people after initial cancer treatment has ended. Survivorship care includes issues related to aftercare; managing delayed side effects of treatment; improving quality of life; and psychological and emotional health. The objective of aftercare is to improve the survival, manage physical symptoms and psychosocial effects, and enhance the quality of life for cancer survivors.
As early as 2014, the European Cancer Patient’s Bill of Rights was published5 by the European Cancer Concord (ECC), a coalition of cancer organisations, patient groups, and healthcare professionals. The Bill of Rights outlines a comprehensive set of fundamental rights that aim to highlight the importance of patient-centred care, and to encourage healthcare providers and policymakers to prioritise the needs and rights of patients with cancer. It includes the right to access information about their diagnosis, treatment, and follow-up care; the right to receive personalised care, and to participate in decision-making about their treatment; the right to be treated by a multidisciplinary team of healthcare professionals; and the right to receive psychological and social support.
The rights of patients with cancer, as described above, have been endorsed by organisations across Europe, such as cancer organisations, patient groups, healthcare professional associations, and governments, including the Greek government. National plans to tackle issues of cancer care are in place in Greece.6,7 As the field of cancer care continues to evolve in Greece, it is crucial to acknowledge the significance of patient preferences, and to consider the acceptability of emerging plans of follow-up care for the expanding population of survivors of cancer. It is essential to ensure that any plans for cancer care align with patients’ preferences, needs, and lifestyles, to promote optimal adherence and outcomes.
This paper presents the qualitative phase, and the protocol for the subsequent stages of a DCE that will be conducted with the aim to identify the preferences of Greek patients with cancer regarding a cancer care model’s key characteristics. To the best of the authors’ knowledge, this will be the first study in Greece to systematically measure and quantify patients’ preferences, offering valuable insights into what patients consider important. By understanding patients’ preferences, the study can identify areas for improving cancer care in Greece, and contribute to more patient-centred models. It aims to identify preferred attributes and quantify trade-offs made by patients in their cancer care decisions.
Importantly, the findings of this study can provide valuable insights for decision-makers in designing cancer care models and facilities. By understanding patient preferences, decision-makers can tailor healthcare system provisions to better meet the needs and expectations of patients with cancer. This, in turn, can lead to improved outcomes in cancer care, the optimisation of resource allocation, and the reduction of healthcare disparities.
METHODS
This study will utilise a DCE to capture patients’ preferences for the attributes of a cancer care model. The DCEs involve presenting participants with a series of hypothetical choices between different alternatives that are described by a set of attributes.8 By varying the levels of the attributes, researchers can assess the relative importance of each attribute to respondents, and the trade-offs they are willing to make between them. The results of a DCE provide a quantitative measure of the importance of each attribute, and can inform policy decisions, resource allocation, and the development of new interventions that align with patients’ preferences.9
DCEs have been used extensively in health economics and outcomes research, particularly in the context of healthcare decision-making.10 They have been employed to assess patient preferences for different treatments, health states, and healthcare services, as well as to evaluate the impact of different factors on healthcare decision-making, such as cost, efficacy, and side effects.11 DCEs have been shown to be a reliable and valid method for eliciting preferences, with high levels of test-retest reliability and construct validity.12 Compared with traditional rating and ranking methods for eliciting preferences in healthcare, DCEs have been found to be more efficient and provide more information about the relative importance of attributes than traditional methods, making them a preferred method for preference elicitation in healthcare research.13
Typically, the development of the DCE survey involves several stages, to ensure that the DCE is developed correctly, and that the data collected are reliable and valid. Figure 1 depicts the steps required for developing a DCE.
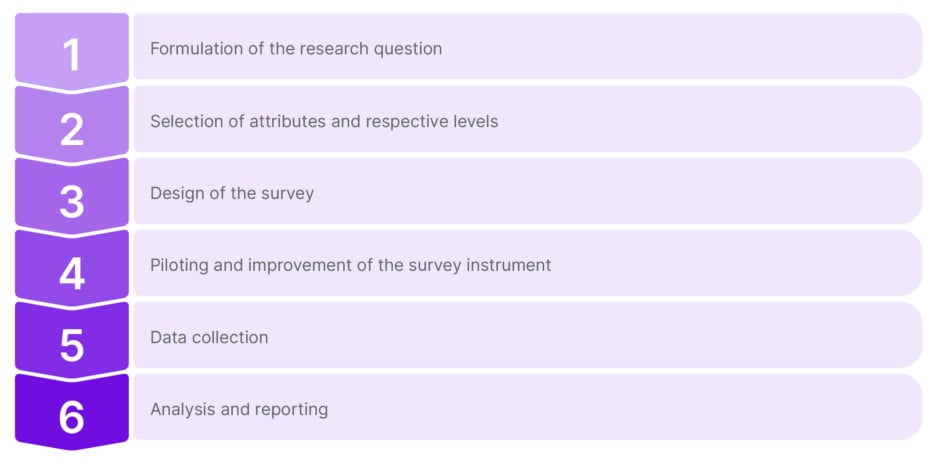
Figure 1: Process for developing a discrete choice experiment.
Formulation of the Research Question
This study was commissioned by the Association of Oncological Patients (KEFI) in Greece,14 an organisation that supports patients with cancer in managing their illness effectively. KEFI offers practical and administrative assistance to ensure that patients with cancer can access the necessary care and support. The association’s mission is to improve quality of life by offering support to navigate the complex healthcare system.
The research question is relevant to the current developments in the healthcare sector in Greece, specifically regarding cancer care. The aim of the study is to capture the preferences of patients with cancer regarding the various characteristics of cancer care in Greece. By exploring patients’ preferences, the study seeks to identify areas for improvement in cancer care, and to ensure that patient-centred care is being delivered. Ultimately, the study aims to contribute to the enhancement of cancer care services in Greece by providing valuable insights into the needs and preferences of patients, and this was established with consultation with the Governing Committee of KEFI.
Selection Of Attributes And Respective Levels
To identify the attributes and respective levels for this DCE, two distinctive but complementary, research methods were followed: targeted literature reviews (TLR) and focus group interviews (FGI), with the patients-members of KEFI.
Targeted Literature Reviews
The aim of the TLR was to create a comprehensive list of potential descriptors for cancer survivor care, along with specific characteristics. To achieve this, separate literature searches (Search 1, 2, and 3) were conducted. The separate searches were conducted in a complementary way, i.e., to be able to identify various aspects of cancer care models via a variety of sources.
Search 1 focused on identifying published papers that described cancer care models in Greece. The study will take place in Greece; hence, this was considered the starting point, and is essential to develop the list of potential attributes for the DCE. Search 2 aimed to identify cancer care models in countries other than Greece. The intention was to enrich the list of potential services for survivors of cancer by identifying services provided in other countries but not in Greece. Since the DCE is based on hypothetical scenarios, it provides the opportunity to capture patients’ preferences for attributes of the services that could potentially be available in the Greek healthcare setting. It was anticipated that the number of identified records for this review would be very large; hence, for this part of the review, the focus was on identifying only systematic or meta-systematic reviews. Search 3 aimed to identify methodologically relevant literature, i.e., DCEs conducted on the same topic, which would assist with the attribute selection.
The literature search resulted in a list of domains that included topics such as healthcare practitioners’ level of expertise; method of contact; location and frequency of follow-up visits; waiting time for booking an appointment, as well as waiting time during the appointment; and the existence of additional supportive or educational activities. While not exhaustive, this list played a pivotal role in shaping the topics for the FGI. Its purpose was to provide a starting point for participants to delve into their thoughts and opinions on the key aspects of cancer care that held the most significance for them.
Focus Group Interview with Patient-Members of KEFI
For the FGI, members of KEFI were recruited. Initially, all members were informed, via email or text message, about the upcoming study, which would involve conducting an FGI. Members were notified with the following message:
“For the first time in Greece, at the initiative of KEFI, a study is being conducted with the aim of eliciting the quantitative preferences of cancer patients for models of care following their initial treatment. We invite you at the premises of our association to participate in the first phase of the study, where there will be a discussion on the needs of the Greek cancer patient. Our association will use the findings of the study to initiate necessary actions to inform and mobilise healthcare policymakers to design better, patient-centred health policies that consider patients’ preferences.”
Approximately 30 members responded to the invitation, showing interest in the study, and willingness to participate in the FGI. On the day of the FGI, 15 members attended the event. Hence, for the FGI, the convenience sampling of 15 participants was deemed appropriate.
After the welcome, introduction, and completion of the consent form, the focus group co-ordinator provided participants with an overview of the focus group’s objectives, and the planned steps. The results of the TLRs were presented, serving as the groundwork for the subsequent focus group discussion. Subsequently, the participants were divided into two groups to discuss the characteristics of a care model that they would ideally like to see implemented in Greece, drawing from their personal experiences and their journeys with their illness. The two groups were moderated by two researchers specially trained in conducting FGIs. Participants were explicitly informed that the list of components served as a reference, and they were encouraged to share and discuss issues based on their unique perspectives, experiences, and preferences for the model of care after the initial treatment. The discussion was conducted using open-ended questions, intentionally without any predetermined order, and, most importantly, without the focus group moderators expressing a position, or influencing the participants’ responses. This approach was designed to facilitate impartial and unrestrained discussions, allowing participants to freely articulate their perspectives and insights. The process was guided by the co-ordinators’ neutrality and objective stance, enabling focus group participants to thoughtfully explore the critical parameters of a care model without co-ordinators’ intervention.
Each of the two groups, following the process described above, produced a distinct list of themes (attributes) characterising a desirable cancer care model. These lists were then merged, and further discussed in the next stage of the FGI, where all the participants agreed on a final list of characteristics (presented in Table 1). All characteristics are deemed to be suitable for the DCE on the basis of three criteria: relevance to the research question, relevance to the decision context, and whether attributes are related to one another.9
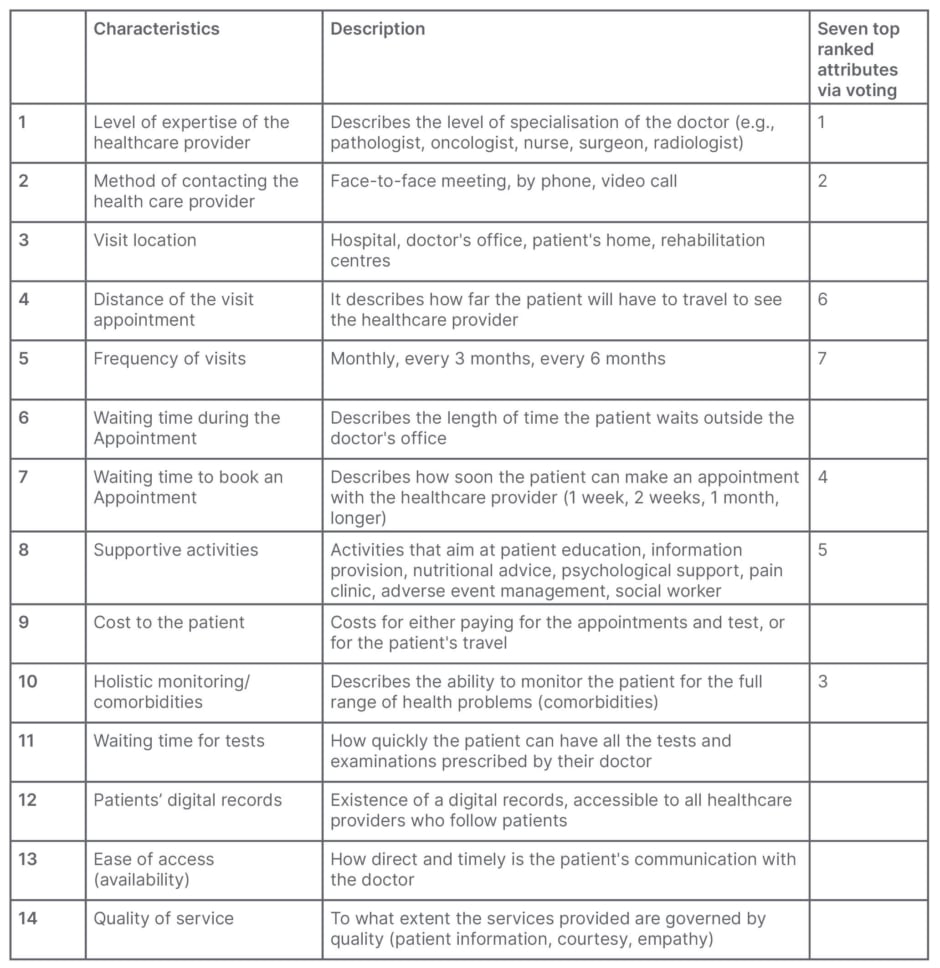
Table 1: List of characteristics for a model of cancer care as produced by the focus group.
Following the identification of characteristics, the participants were asked to rank them by order of importance via an online voting system, where Google forms were utilised. The objective of this task was to determine the top five to seven attributes that could be utilised in the DCE. According to the existing literature on DCEs, the average number of attributes in published studies is estimated to be 5.74 (standard deviation: 1.98), with a minimum of two and a maximum of 12.15 Therefore, adhering to common practice in DCE development, the authors considered a range of five to seven attributes to be satisfactory, ensuring that the DCE would not impose excessive cognitive demands on the respondents.16 The seven top ranked attributes are presented in the last column of Table 1.
The findings of the FGI were further discussed with the Governing Committee of KEFI in a final stage of the qualitative part of the study, where a definitive list of attributes was created to develop the DCE survey (Table 2). The levels for each attribute were informed by a literature review applicable to the Greek context, and qualitative interviews with the Governing Committee of KEFI. Out-of-pocket costs to patients were additionally included, as this was deemed important due to the healthcare market dynamics in Greece, which involves a mix of private and social insurance coverage. In this context, it is common for patients to incur routine out-of-pocket medical expenses for procedures and/or medical tests. Additionally, by incorporating out-of-pocket costs as an attribute, it allows the authors to use it as a standardised measure to evaluate the trade-offs that respondents make when assessing various attributes of a cancer care model.
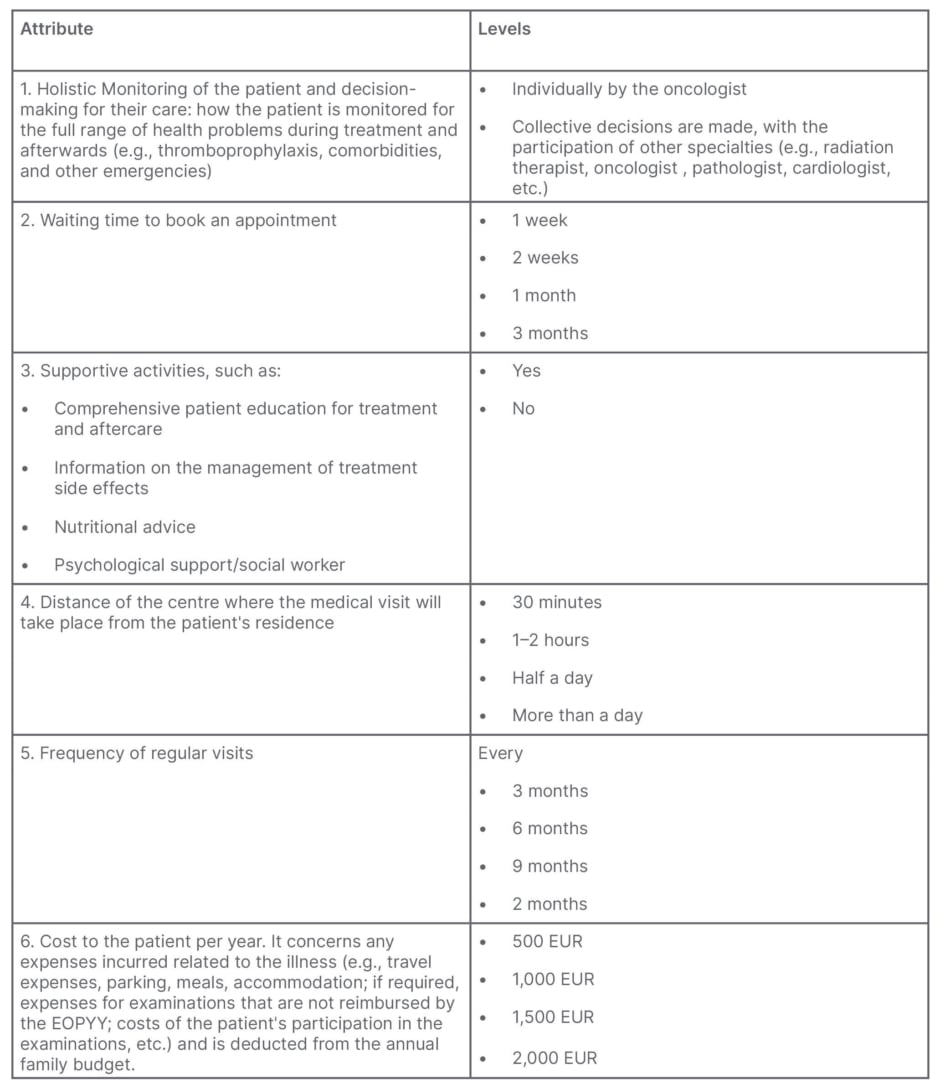
Table 2: Final list of attributes and levels included in the discrete choice experiment.
EOPPY: National Organization for Health Care Services.
Design of the Survey
Following the identification of attributes, the next step involves the experimental design for the DCE. Given the large number of profiles resulting from the combination of attributes and levels (2^[2]×4^4=1024 profiles), which result in 523,776 possible choice sets, a full factorial design is not feasible. Instead, an efficient experimental design will be constructed using the relevant macros in the SAS® statistical software (SAS, Cary, North Carolina, USA).17 Efficient experimental designs in the context of choice modelling refer to designs that optimise the precision of estimated parameters, while minimising the number of choice questions or choice sets presented to participants.18 The goal is to gather the maximum amount of information with a minimal number of choice tasks, reducing participant burden, and saving resources. Additionally, to serve this aim, a blocked design might be adopted.
In addition to the DCE questionnaire, the survey will include questions covering four main domains:
• Sociodemographic data on age, gender, education, occupation, and income, to provide context and possibility for subgroup analysis.
• Disease-related data on the type of cancer and time since diagnosis, to account for variations in preferences.
• Cancer care in Greece, enquiring about treatment options, access to healthcare services, provider interactions, and challenges, to understand the current state of cancer care in Greece.
• The EuroQol19 EQ-5D-5L questionnaire to allow for the assessment of the respondents’ current health state, across five dimensions (mobility, self-care, usual activities, pain-discomfort, and anxiety-depression). The EQ-5D-5L questionnaire is a standardised instrument designed to assess health-related quality of life. It is widely used in health research, clinical studies, and health economics.
Through these additional questions, the survey aims to collect a comprehensive dataset, beyond the DCE choices, which allows for a nuanced analysis of participants’ preferences concerning their sociodemographic characteristics, disease-related factors, healthcare experiences, and current health status. It provides a richer context for interpreting DCE results in the context of cancer care in Greece.
Piloting and Improvement of the Survey Instrument
The final survey instrument will be piloted with a small sample from KEFI members (approximately 25–30), who represent this study’s target population. This step is crucial to ensure the survey’s validity, reliability, and clarity. During the pilot phase, participants’ feedback on their understanding of the questions, and any challenges they encounter while completing the survey, will be gathered and analysed. In addition, the length of the survey and the time required for participants to complete it will be tested. Long surveys may lead to respondent fatigue, and a higher likelihood of incomplete or rushed responses. The piloting of the survey will lead to iterative improvement, as the survey instrument will be revised accordingly, based on the pilot test findings and participant feedback. Once the revisions are made, the final survey instrument will be deployed.
Data Collection
The survey will be conducted online, allowing respondents to complete it at their convenience via a web browser. This approach offers flexibility and convenience, enabling participants to complete the survey from any location with Internet access. The administrative team of KEFI will play a central role in recruiting participants, members of KEFI, or other associations in Greece set up for patients with cancer, for the survey. The primary criterion for participation is that respondents have been, or are currently, patients with cancer. No additional inclusion criteria will be applied to ensure an adequate number of responses.
Before beginning the survey, participants will be presented with a patient information sheet detailing the study’s purpose, procedures, confidentiality, and their rights as participants. They will then be asked to provide informed consent before proceeding with the survey.
The determination of the final sample size for a DCE considers various factors specific to the design itself. These factors include the number of attributes, the range of attribute levels, the number of choice scenarios, the number of alternatives within each choice set, and the influence of prior parameters.20 Prior DCEs conducted in this field show a variability in the number of respondents, as well as the number of attributes that were used in the DCE: 185 respondents for a DCE with six attributes,21 668 respondents for a DCE with eight attributes,22 722 respondents for a DCE with five attributes,23 and 331 respondents for a DCE with five attributes.24 In this study, the final number of respondents required for the study will depend on the final experimental design.
Data Analysis, Reporting, and Dissemination
DCE data analysis typically involves regression modelling within a random utility framework. The random utility model is a commonly used approach in DCE analysis, and is based on the Lancaster theory of consumer demand.25,26 This framework assumes that individuals make trade-offs when making decisions, and choose the option that provides the greatest utility, determined by the attributes associated with the product or service. The utility of each alternative can be decomposed into systematic (observable) and random (unobservable) components (Figure 2).27 The systematic component represents the part of utility that can be explained by the attributes and their levels. It is typically modelled using regression techniques, such as the conditional logit, mixed logit, or latent class models. These models estimate the relationship between the attributes and the choice probabilities, allowing for the calculation of attribute importance, and the assessment of trade-offs. The random component represents unobserved factors or individual-specific heterogeneity that affects the decision-making process. It is assumed to follow a specific distribution, such as the extreme value distribution, in the case of the conditional logit model.28 This component captures idiosyncratic preferences, taste variations, and random errors in decision-making.

Figure 2: Equation.
Based on the above, the analysis will start with a multinomial logit model,29 which is commonly used in the analysis of DCE data. The model estimates the parameters that quantify the relative importance of the different attributes in influencing the choice probabilities. Further, other extensions of this model,30 such as the random parameters logit model or the mixed logit model, and latent class models, will be explored to capture individual heterogeneity, and account for preference variation across the sample. These models relax the assumption of identical preferences across individuals, and allow for more flexible estimation of preference heterogeneity.
Measures of goodness of fit, such as log-likelihood, McFadden’s R squared, Ben-Akiva-Lerman; and information criteria, like Akaike information criterion and Bayesian information criterion, will be used to determine the final parsimonious model in the context of choice modelling.
The study results will be disseminated via national and international scientific and health policy-related conferences to the academic community, policymakers, and the general public. It is envisaged that a manuscript will be published in a peer-reviewed journal.
SUMMARY
This study reports on the development of the qualitative phase of a DCE, and describes a comprehensive protocol for the subsequent phases of the study. The utilisation of the DCE approach in the authors’ study holds promise for capturing the patient preferences regarding models of care for survivors of cancer in Greece, aligning with the global paradigm shift towards patient-centric care models. The findings of the study will enhance healthcare policymakers’ and clinicians’ understanding of patients’ needs and preferences, and subsequently develop strategies for the improvement of care for patients with cancer. The utilisation of a DCE reflects the broader trend of employing DCEs in healthcare decision-making, contributing to the growing literature on eliciting patient preferences.11
The aim of the qualitative phase of the study was to identify the attributes and levels which will be utilised in the experimental design of the DCE. A rigorous qualitative approach, which involved literature reviews, qualitative interviews, and FGI, was employed. Further, the large number of attributes which resulted from the FGI was scaled down to a manageable number by a rating exercise. This systematic combination of methods enhances the study’s quality and validity.12 Nonetheless, this study faces methodological limitations inherent in all DCEs. For instance, the utilisation of convenience sample for the FGI may be perceived as a potential source of bias, as the participants may not fully represent the diversity of perspectives within the broader population.
The DCE will be completed by a broad and diverse sample of individuals who have undergone, or are undergoing, cancer treatment. The study will provide insights into the patients’ trade-offs for the different attributes of cancer care, aiding policymakers in understanding the preferences of patients. These study findings will be placed in the context with existing research on how patients prefer post-cancer care, to gain a broader perspective of the general preferences for their care.







