Abstract
The recent emergence of the Nipah virus in the same district of Kozhikode, India, following its outbreak in 2018 and 2021, has elicited heightened apprehension among the public amidst the era of COVID-19. The potential fatality associated with this virus has been effectively mitigated through mass awareness, community and healthcare involvement, and stringent implementation of preventive measures. Nevertheless, the rate of transmission has consistently escalated over a span of several years, suggesting an emerging potential for global public health implications. Although the transmission rate remains low, the elevated mortality linked to the Nipah virus constitutes a potential threat, accentuated by the absence of vaccines and dependable treatments, thereby underscoring the risk to public health and emphasising the necessity for proactive measures to protect the wellbeing of the community. This narrative review provides an overview of the Nipah outbreaks in Kerala, India, and its global impact by conducting a thorough search of databases such as PubMed, Google Scholar, and ResearchGate using the following keywords: “Nipah virus,” “Henipavirus,” and “Kerala.”
Key Points
1. Recurrent Nipah outbreaks in Kerala, India, emphasise the critical need for preparedness due to its high fatality rate and potential for human transmission, underscoring the urgency of proactive measures to prevent it from emerging as a pandemic.2. This article comprehensively analyses Nipah virus outbreaks, focusing on epidemiology, clinical features, preventive measures, treatment, public health responses, and the ‘One Health’ approach, from Kerala-specific data.
3. Healthcare professionals must prioritise early recognition of Nipah virus symptoms, enhance surveillance efforts, engage in interdisciplinary collaboration, and advocate for public awareness to mitigate future outbreaks and safeguard public health.
BACKGROUND
Nipah virus disease is a zoonotic infection caused by the Nipah virus (NiV) of the Genus Henipavirus of the Paramyxoviridae family, an enveloped ribonucleic acid virus. The natural host of the virus is supposed to be the Pteropus bats (fruit-eating species, popularly known as flying foxes).1 According to the World Health Organization (WHO), the fatality rate of NiV is estimated to be high, ranging from 40–75%. This indicates that NiV is significantly more lethal compared to COVID-19, which exhibits a mortality rate ranging from 0.1–19.0%, depending on the local capacity for epidemiological monitoring and healthcare management.2,3 Unlike the previous viral outbreaks, the incidence of NiV infection has been comparatively limited, with a reduced occurrence of human-to-human transmission. Yet, it is intriguing that NiV infection exhibits a significantly higher fatality rate, suggesting the potential for future outbreaks to occur.4
METHODS
The authors conducted an extensive search across PubMed, Google Scholar, and ResearchGate, focusing on articles written in English by using keywords such as “Nipah virus,” “Henipavirus,” and “Kerala,” primarily targeting studies published from 2018 onwards, coinciding with the first reported case in Kerala, India. The authors gathered information on various aspects of NiV, including its epidemiology; outbreaks in countries such as Malaysia, Singapore, Bangladesh, India, and other countries; as well as covering clinical manifestations, diagnostic methods, preventive measures, treatment options, vaccine development, public health interventions, surveillance strategies, and the economic impact associated with NiV infection. The findings regarding NiV were compiled from reputable scholarly journals and authoritative public health sources.
EPIDEMIOLOGY
NiV was initially reported in Malaysia and Singapore between 1998–1999 and caused severe febrile encephalitis among pig farmers. The transmission of the virus to humans occurs through direct contact, inhalation, or ingestion of contaminated food with NiV, which was facilitated by infected bats and infected pigs as intermediate animal hosts. The outbreaks resulted in the deaths of 105 individuals, leading to mass panic and notable socio-economic upheaval.5 After the initial occurrences, subsequent outbreaks were documented in Meherpur, Bangladesh, followed by Siliguri city, West Bengal, India, in 2001.3 The mode of transmission, clinical manifestations, and mortality rates were different in Indo-Bangladesh when compared to the outbreak in Malaysia. The significant aspects of this outbreak were the transmission of the virus between individuals, and nosocomial infections. Furthermore, the Indo-Bangladesh outbreak exhibited rapid progression of the disease with high secondary attack rates and severity of the disease. The probable reason for the high mortality could be acute respiratory distress syndrome and respiratory failure accompanied by multi-organ dysfunction syndrome, along with neurological manifestations.3,6 Multiple small outbreaks have been reported in Cambodia, Indonesia, Ghana, Madagascar, the Philippines, and Thailand. However, outbreaks in Malaysia (43%), Bangladesh (42%), and India (15%) contributed to the overall global incident cases.7 The outbreaks reported in Cambodia provide limited insight into the circulation of bat-borne diseases, including NiV. Although the virus was isolated from a roost of Pteropus lylei bats in Western Cambodia in 2000, this discovery remains unverified, and there have been no documented cases of NiV infection in humans within the country to date.8 Similarly, in Thailand, where NiV circulates in P. lylei populations, no human or domestic animal cases have been reported.9 Epidemiological evidence from the Philippines in 2014 indicates that the primary mode of NiV transmission to humans involved direct contact with infected horses, exposure to their contaminated bodily fluids during the slaughtering process, and/or ingestion of inadequately cooked meat from diseased horses. However, clinical and epidemiological data suggest instances of direct human-to-human transmission in at least five cases.10
Nipah Outbreak in Kerala
Over the last 5 years, Kerala has grappled with four Nipah outbreaks, the most recent occurring in 2023. These incidents, spanning 2018, 2021, and 2023, have primarily affected the Kozhikode district and its surrounding areas, with an additional occurrence in 2019 in the Ernakulam district.11 Table 1 shows the major findings during the Nipah outbreaks in Kerala, and Figure 1A and 1B illustrate the geographical distribution of Nipah outbreaks, detailing cases and deaths in Kerala. The strain of NiV found in Kerala exhibited a genomic sequence sharing a resemblance ranging from 85.14–96.15% with the M and B genotypes of NiV found in Malaysia and Bangladesh.17 In 2018, all cases in Kerala, excluding the index case, experienced human-to-human transmission, with exposure potentially occurring during activities such as cleaning a bat-infested unused well or visiting the nearby forest. The mapping of fruit bats in the area and specimen studies confirmed the presence of the virus in the bats captured by animal husbandry and national agencies.12 The National Institute of Virology, Pune, India, confirmed in 2021 that antibodies detected in bat samples belonged to the Rousettus genus, in addition to the previously identified Nipah strains in the Pteropus genus.18 The State Government of Kerala documented six laboratory-confirmed cases of NiV infection, in two fatalities, among males aged 40 and 49 years, from 12th–15th September 2023. The present cases are epidemiologically connected to the initial case with an unknown source of infection, focusing on the Kozhikode district.11 The expeditious urbanisation and environmental alterations in Kerala may contribute to Nipah outbreaks by disrupting natural habitats, which prompts bats to migrate closer to human populations.11,16 The region’s climate change, evident through rising temperatures and altered rainfall patterns, is thought to amplify viral epidemics by fostering conditions conducive to increased virus transmission. Other contributing factors to the recurrent spillover of NiV include extensive forest fragmentation, elevated livestock density, industrialisation, and concentrated human settlement in Kerala.7,19
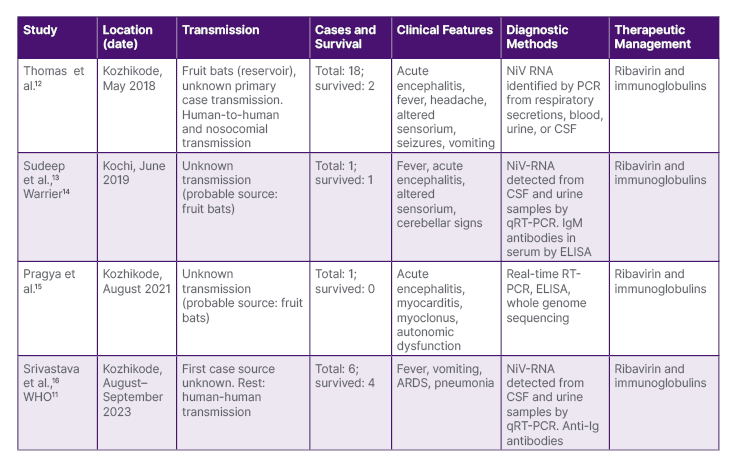
Table 1: Major findings during the Nipah outbreaks in Kerala.
ARDS: acute respiratory distress syndrome; CSF: cerebrospinal fluid; ELISA: enzyme-linked immunosorbent assay; NiV RNA: Nipah virus ribonucleic acid; qRT-PCR: quantitative real-time reverse transcription; RT-PCR: reverse transcription polymerase chain reaction.
CLINICAL FEATURES OF NIPAH VIRUS AND ITS DIAGNOSIS
Variations in the incubation period of NiV have been observed across multiple geographical regions. Generally, the incubation period of NiV in Kerala ranges between 4–21 days.20 However, prolonged incubation periods have also been noticed, particularly the outbreak in Malaysia, where the incubation period was reported to be up to 2 months.21 Individuals affected with the viral infection show a spectrum of conditions, ranging from asymptomatic subclinical infection to the development of acute respiratory disease, and potentially deadly encephalitis. Contrary to Malaysia, the outbreaks in Bangladesh and India do not include any information about asymptomatic NiV infection.6 The major clinical manifestations commonly observed in individuals infected with NiV include respiratory distress, nausea, vomiting, headache, fever, and severe encephalitis. Other symptoms reported were behavioural distortion, confusion, pneumonia, reduced consciousness, muscle pain, diarrhoea, cough, and disruptions in the nervous system.3,20 Detecting NiV infection in its initial phases can pose challenges due to the existence of non-specific early symptoms linked to the illness. Nevertheless, the timely identification and assessment of cases assume paramount importance in enhancing the likelihood of survival among affected individuals, curbing the spread of infection to others, and effectively coordinating response measures during an outbreak. Various diagnostic tests are available for the identification of NiV infection. Diagnostic procedures like real-time PCR (RT-PCR) on samples obtained from throat and nasal swabs, urine, cerebrospinal fluid, and blood can be employed during the initial stage of the disease. Adequate and proper infection control measures should be adopted during sample collection, transportation, storage, and processing of the samples of the suspected patients to avoid further risks. The enzyme-linked immunosorbent assay (ELISA) is used to test for antibodies later in the course of the illness and following recovery.3,20,22
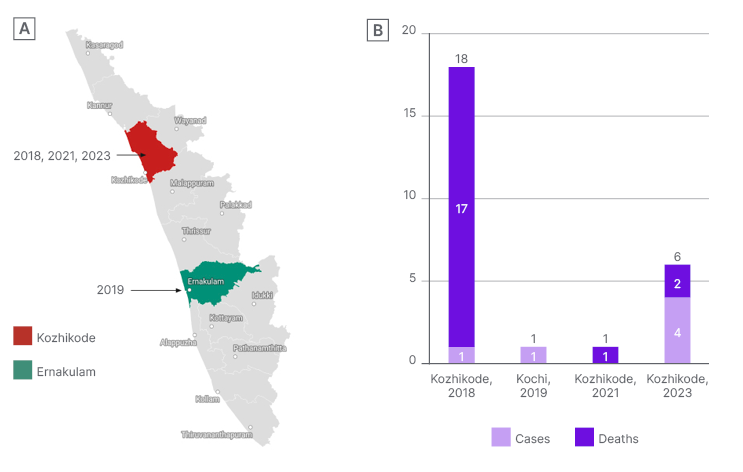
Figure 1: A) Geographical distribution of the Nipah outbreaks in Kerala, India; and B) year-wise Nipah virus cases and deaths in Kerala.
PREVENTIVE MEASURES
Although past NiV outbreaks were confined to localised areas, their inherent lethality elicits apprehension among researchers expressing concerns about the prospect of increased human-to-human transmission augmenting the virus’ contagiousness.16 Hence, it is imperative to implement suitable preventative and control strategies to prevent any further transmission of NiV, which includes practising regular handwashing, employing 70% ethanol sanitisation, and refraining from sharing things with infected individuals.16,23 As bats are the natural reservoir hosts of NiV, vigilant inspection of fruit from bat-inhabited trees, and surveillance in affected areas are critical for early outbreak detection. Strict adherence to handwashing, usage of personal protective equipment like gloves and face masks, and the isolation of the affected patients can mitigate health worker transmission.11,16,20,23 Prompt isolation of symptomatic individuals and dedicated NiV wards with discharge contingent on negative RT-PCR results are essential. Discharged patients should be isolated for 21 days from infection detection due to the unclear incubation period.11,20 Community awareness campaigns and utilising various media platforms can help educate the public about the risk factors and stress the importance of strict preventive measures to contain virus spread, particularly within socioeconomically disadvantaged populations.11,20,24
TREATMENT
The Nipah viral infection exhibits distinct characteristics compared to other viral diseases, due to its ability for human-to-human transmission, with no specific treatment or vaccines available to date. Therefore, effective management primarily encompasses the implementation of infection control measures, the process of triaging, the practice of isolation, and the comprehensive management of patients, which includes providing intense supportive care.11,20 During previous outbreaks, the administration of ribavirin and acyclovir has been employed as a therapeutic intervention for the treatment of NiV infection. The mortality rate was reduced to 36% when ribavirin was administered orally or intravenously during the Malaysian outbreak, whereas acyclovir was utilised during the outbreak in Singapore. However, the effectiveness of these treatments remains uncertain.25,26 Evidence-based studies on the efficacy of the antiviral drug favipiravir (T-705) in hamsters infected with NiV have exhibited positive outcomes, along with investigations into vaccines using the hamster models.27 It is essential to develop targeted antiviral therapeutics to tackle the deleterious impact of NiV on the public as early as possible.
PUBLIC HEALTH RESPONSE IN KERALA
In response to the outbreak, national and state authorities, including the Department of Health and Family Welfare (Government of Kerala), the National Centre for Disease Control (NCDC), the Indian Council of Medical Research (ICMR), the National Institute of Epidemiology (NIE), the National Institute of Virology (NIV), the National Institute of High Security Animal Disease (NIHSAD), and the State Animal Husbandry, promptly implemented a comprehensive multisectoral coordination and response strategy with support from the Ministry of Health and Family Welfare (MoHFW).11,28,29 This involved intensified surveillance, contact tracing, and laboratory testing for suspected cases and high-risk contacts, ensuring hospital readiness for case management, and robust risk communication and community engagement. Containment measures in the affected area included the enforcement of restricted zones, movement restrictions, social distancing, and mandatory mask-wearing. The state government meticulously followed a standardised protocol for managing and transferring deceased individuals, utilising dedicated ambulances with skilled medical personnel. Additionally, the implementation of point-of-care assays, monoclonal antibody administration, bio-risk management, and hospital infection control training for healthcare workers significantly contributed to the effective control and containment of the NiV outbreak.2,11,28,29
NIPAH AS A POTENTIAL THREAT
The global impact of COVID-19 serves as a poignant reminder of the necessity for proactive and collaborative efforts in addressing global health crises. The long-term consequences of the pandemic have been felt across various sectors, including health, education, and the economy. This highlights the critical lesson that a coordinated and pragmatic approach is essential to manage and mitigate the repercussions of future outbreaks effectively.30
Drawing parallels to historical outbreaks, the 1998 NiV outbreak in Malaysia serves as a pertinent example. Beyond the immediate costs associated with culling livestock, this event triggered extensive economic repercussions. The long-term aftermath encompassed disruptions in international trade, tourism, agriculture, and travel. The collapse of the pig farming industry led to widespread unemployment and underemployment. The overall economic impact of the 1998 Nipah outbreak in Malaysia was estimated at a staggering 582 million USD.31,32 While the transmission modes of NiV in India may differ from those in Malaysia, the lessons learned from historical outbreaks emphasise the need to prevent and manage such events effectively. The mortality, morbidity, and economic losses associated with outbreaks make it crucial to establish global collaborative mechanisms to respond swiftly and comprehensively.
Moreover, Kerala has been susceptible to a spectrum of health threats, including mpox and COVID-19, stemming from its global connectivity, international trade, and a significant diaspora. The state’s extensive international links and a substantial overseas healthcare workforce contribute to its vulnerability.33 Additionally, zoonotic diseases such as rabies, leptospirosis, Kyasanur forest disease, and bird flu (avian influenza) continue to pose significant risks in Kerala.34 Unlike COVID-19, which easily spreads among humans, zoonotic diseases often have a specific animal host before infecting humans. This limited host range reduces their pandemic potential if adequate precautious measures are taken. Transmission modes, such as direct contact with infected animals or their fluids, can hinder widespread human-to-human spread, unlike respiratory viruses like COVID-19. Environmental factors like climate and habitat also influence the spread of zoonotic diseases. Some pathogens are confined to specific ecological niches or regions, limiting their global impact.35
The recent Nipah outbreak in Kerala exemplifies the risks, with unknown exposure in the initial case, higher incidents of human-to-human transmission, limited understanding of virology, and a lack of specific treatment and vaccination. NiV demonstrates a notable propensity for mutation, enabling it to adeptly acclimate to varied environments, infiltrate new hosts, develop novel virulence mechanisms, evade human immune responses, and potentially amplify its transmissibility. If the virus were to mutate in a way that enhances its ability to transmit between humans or evade the immune system, it could increase the likelihood of a pandemic.36 Therefore, extreme precautions should be taken to safeguard against future outbreaks in Kerala, and mitigate risks beyond its borders by enhancing surveillance systems, early detection, developing vaccines and specific antiviral therapies, fostering enhanced public awareness, and leveraging advanced technologies like digital health.
ONE HEALTH CONCEPT
The cross-species transmission of the disease, extending to humans, emphasises the critical need for a ‘One Health’ approach, demanding inter-sectoral collaboration at the global, national, state, and district levels. This strategy, especially in the context of zoonotic diseases like that of NiV, seeks to institute an integrated coordination mechanism with consideration of the human-animal-environment interface, while leveraging the extant surveillance systems of multiple diverse stakeholders for the early detection of potential outbreaks (Figure 2). This collaborative framework enables a prompt and efficient public health response, fostering the exchange of pertinent information among stakeholders to facilitate necessary actions.29 Kerala was able to confine the further spread of NiV by adopting a comprehensive ‘One Health’ approach, which facilitated seamless coordination among health, veterinary, environmental, and public health sectors.
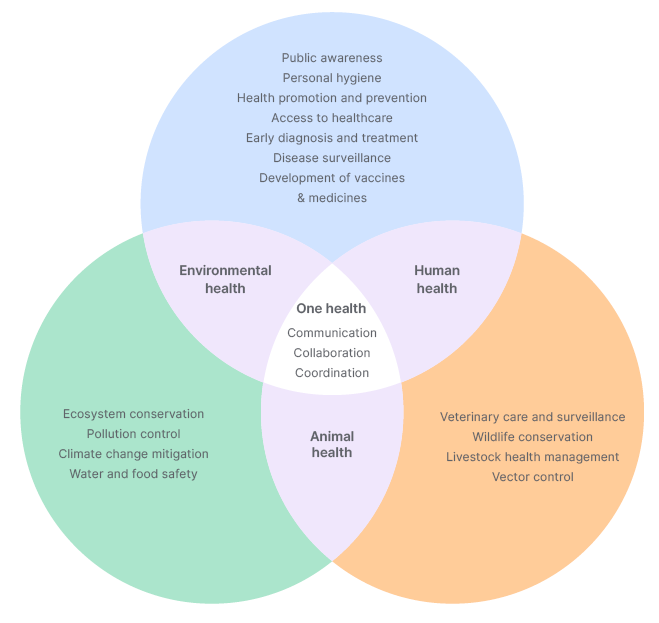
Figure 2: One Health approach against Nipah virus.
CLINICAL RELEVANCE AND RECOMMENDATIONS
The emergence of NiV outbreaks in Kerala and its recurring nature underscores the urgent need for heightened clinical awareness, and preparedness among healthcare professionals. Given the high fatality rate associated with NiV infection, early recognition of the symptoms, and prompt diagnostic testing are imperative for timely intervention and containment of the disease spread. Clinicians must remain vigilant, particularly in regions with a history of NiV outbreaks, to promptly identify suspected cases and initiate appropriate infection control measures.
Furthermore, the diverse clinical manifestations of NiV infection, ranging from respiratory distress to neurological complications, necessitate a comprehensive approach to patient management. Healthcare providers should be equipped with the necessary resources and expertise to provide intensive supportive care tailored to the individual’s needs. Additionally, the absence of specific treatments or vaccines for NiV highlights the importance of symptom management and supportive therapy in improving patient outcomes.
RECOMMENDATIONS
Enhanced surveillance: implementing robust surveillance systems for early detection and monitoring of NiV outbreaks is paramount. This includes regular monitoring of bat populations, conducting serological surveys in high-risk areas, and maintaining vigilance for unusual clusters of febrile illness or encephalitis cases.
Capacity building: healthcare facilities should be adequately equipped and staffed to manage NiV cases effectively. This includes training healthcare workers in infection control protocols, case management, and the safe handling of laboratory specimens.
Public awareness: engaging in community outreach and education initiatives is essential for raising awareness about NiV transmission, symptoms, and preventive measures. Empowering communities with accurate information can facilitate early recognition of symptoms, and encourage adherence to preventive practices.
Research and development: continued investment in research, aimed at developing vaccines and specific antiviral therapies for NiV, is crucial. Collaborative efforts between government agencies, research institutions, and pharmaceutical companies can accelerate progress towards effective prevention and treatment strategies.
One Health approach: adopting a ‘One Health’ approach that integrates human, animal, and environmental health considerations is essential for mitigating the risk of future NiV outbreaks. This interdisciplinary approach facilitates early detection, rapid response, and coordinated interventions across sectors.
CONCLUSION
The resurgence of the Nipah outbreak, in the same location, in Kerala, should be monitored to avoid potential causes of future occurrences, by implementing preventive measures, enhancing medical preparedness, and protecting public health against the deadly virus. The knowledge acquired from past outbreaks provides insights into formulating an effective approach. This not only protects the affected area but also serves as a paradigm for global health security in response to emerging infectious threats.







