Abstract
Superior vena cava syndrome (SVCS) results from the obstruction or narrowing of the superior vena cava, causing venous congestion and various symptoms such as facial and upper limb swelling, shortness of breath, chest pain, coughing, and, in severe cases, dizziness and headache. The primary treatment for SVCS is balloon angioplasty with endovascular stenting. Post-procedural complications are influenced by factors such as SVCS aetiology, comorbidities, and the presence of arteriovenous fistulas. This review examined eight clinical studies to assess the effectiveness of percutaneous endovascular stenting and associated complications, focusing on improving patient prognosis. The research, conducted through internet search engines and reputable databases, revealed that percutaneous endovascular stenting demonstrated efficacy ranging from 95–100% in addressing SVCS. Common complications post-procedure included SVC narrowing recurrence, airway constriction, and mortality, often linked to malignancy. The findings emphasise the need to refine therapeutic approaches, especially in addressing the root cause of SVCS, which is frequently malignancy. Consequently, implementing additional protocols to reduce the risk of SVCS development is crucial. This comprehensive review provides insights into the effectiveness of endovascular stenting in treating SVCS, highlighting the importance of tailored approaches and ongoing efforts to enhance patient outcomes.
Key Points
1. Superior vena cava syndrome (SVCS) presents as clinical manifestations of obstructed venous blood flow to the heart and is often caused by malignancy. Endovascular stenting effectively treats this, yet post-surgery cardiac complications are documented. The treatment’s success remains debatable despite its clinical significance.2. An analysis of eight research papers evaluated the efficacy and complications of endovascular stenting for SVCS. Most studies reported 95–100% efficacy, yet participants often experienced post-operative complications such as airway stenosis, stent dysfunction, and, in some cases, mortality.
3. Whilst endovascular stenting effectively treats SVCS, current literature highlights the necessity for further research into tailored interventions to minimise associated complications. Furthermore, additional investigation to address the primary cause, often malignancy, is needed.
INTRODUCTION
Superior vena cava syndrome (SVCS) is a group of clinical manifestations, caused by an obstruction of venous blood flow to the heart. Normally, the superior vena cava (SVC) drains deoxygenated blood from the upper part of the body into the right atrium of the heart. This disease has been observed and diagnosed amongst many people presenting with characteristic symptoms, with an estimated incidence globally ranging from 1/650–1/3,100 patients.1 The symptoms of SVCS vary, depending on the degree of vasculature compromise by a given cause of obstruction, the rate at which venous blood flow through the SVC is impaired, and the specific underlying cause. Common symptoms include facial swelling, visible enlargement of neck and chest veins, shortness of breath, cough and hoarseness, swelling of the arms, and rarely headache and dizziness indicating central nervous system involvement due to cerebral oedema.
The underlying pathology for SVCS is venous flow obstruction and venous congestion. This can be caused by external compression, and internal occlusion or stenosis of the SVC (most commonly by a malignancy). While malignancy is the predominant factor responsible for approximately 80% of cases of SVC obstruction, there has been a recent rise in the occurrence of cardiac device-related SVCS caused by central venous catheters, as well as pacemaker or defibrillator leads.2,3 Interventions that can lead to SVCS include procedures related to the chest or mediastinum, such as lung resection; mediastinal tumour removal; catheter placement for large or bulky cardiac devices, pacemakers, and implantable cardioverter-defibrillators; or lead placement, which may compress the SVC and contribute to SVCS symptoms. The increasing use of cardiac devices accentuates the need for careful patient selection, monitoring, and prompt evaluation of symptoms suggestive of SVCS.4
The SVC is a crucial part of our low-pressure venous system, and its walls are relatively thin, making them vulnerable to damage by various pathological processes. These processes can be categorised into three main types: compromised vessel anatomy, impaired venous flow, and reduced vessel wall integrity. It is common for patients with SVCS to experience a combination of these three mechanisms at one given moment in time.1 Recognising the significance of SVCS, one can observe and categorise the extent of damage that is placed upon the SVC. Thus, the Stanford and Doty classification for SVC obstruction, a widely recognised system used to categorise different types of SVC obstructions based on anatomical localisation and predominant aetiology, is an important tool.4
A comprehensive clinical assessment is often conducted to diagnose SVCS, beginning with a physical examination to evaluate the degree of venous distention and swelling. Furthermore, diagnostic imaging techniques including chest X-rays, CT scans, or MRI are often utilised to identify the underlying cause of SVCS and assess the extent of the obstruction.1 Neglecting SVCS can lead to other complications that may further hinder a patient’s condition and life expectancy. These complications include, but are not limited to, cerebral oedema, which may often lead to various neurologically associated symptoms such as headaches, confusion, seizures, or even coma. Additionally, oedema of the larynx and upper respiratory tract may concomitantly occur, leading to difficulty in swallowing, voice hoarseness, and, potentially, a compromised airway. Furthermore, reported complications often arising in patients with SVCS include altered respiration, which leads to breathing difficulties and pleural effusion.5
Persistent SVCS may also impede the efficiency of central venous access for post-transplant care and monitoring, potentially leading to further complications or delays in management.6 These complications highlight the importance of a thorough assessment and a multidisciplinary approach involving cardiologists, radiologists, and surgeons to ensure timely diagnosis and appropriate management of this rare but potentially severe complication. Despite the clinical significance of persistent SVCS in patients receiving cardiac transplant, the literature on this specific topic remains limited and fragmented.6
Treatment of SVCS involves both supportive and definitive therapy. Supportive measures include elevating the patient’s head to reduce hydrostatic pressure and oedema, although the effectiveness of this manoeuvre is not well-documented. Glucocorticoid therapy, such as dexamethasone, is commonly prescribed, but its effects have not been extensively studied. Loop diuretics may also be used, but their impact on venous pressure is unclear.6 This systematic review seeks to address the dearth of knowledge regarding cardiac transplant complications in patients with persistent SVCS. By consolidating the available evidence, this review hopes to contribute to the understanding of this complex condition and inform clinical decision-making for improved patient outcomes.
METHODOLOGY
In April 2023, the objectives in this document were provided utilising the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (Figure 1) and Preferred Reporting Items for Overviews of Reviews (PRIOR) guidelines, which were followed rigorously to ensure the transparency and reproducibility of this investigation. This systematic review aims to provide valuable insights into this complex, understudied area, and analyse the effectiveness of endovascular stenting in patients with SVCS, as well as post-procedural complications.
Information Sources and Search Strategies
The studies in this review were selected to provide comprehensive information regarding the results of treating patients with SVCS using endovascular stenting. Specific keywords were interpolated, such as “Superior Vena Cava, SVC, Superior Vena Cava Syndrome, Angioplasty, and endovascular Stenting,” into designated search engines, including Cochrane Library, Google Scholar, and Medline/PubMed. The target population for each study comprised adults aged 18–75 years, with all participants being either male or female.
Several different study designs were considered, including randomised clinical trials (RCT), cohort studies, and retrospective studies. The reviewers of this paper specifically selected papers pertaining to research conducted between 2016–2023, providing a comprehensive and up-to-date pool of relevant studies for the review process. This careful and thorough methodology allowed for a robust evaluation of long-term complications in patients with SVCS treated by endovascular stenting.
Limitations
To evaluate the long-term outcomes of stenting and problems linked to SVCS, adequate follow-up data should have been additionally provided, with there being either a minimum follow-up duration or a predetermined number of follow-up appointments.
Inclusion Criteria
Studies included in this review had patients with a confirmed clinical diagnosis of SVCS, validated by suitable imaging methods. Medical records for patients had to be thorough and well-documented, including pertinent clinical data, imaging results, lab results, and cardiac studies.
The authors decided to include studies discussing SVCS without discriminating based on the underlying aetiology, including malignancy and non-malignant reasons, such as thrombosis, aortic aneurysm, vasculitis, arteriovenous fistulas, and infections (histoplasmosis, tuberculosis, syphilis, and actinomycosis).
Exclusion Criteria
Patients with pre-existing cardiac diseases or conditions unrelated to SVCS were not included in this systematic review to ensure that the research specifically addressed the authors’ research question. To ensure that complete information was available, studies with insufficient follow-up data were disregarded. Additionally, to avoid the introduction of additional confounding variables, patients with comorbidities that significantly influence cardiac function, such as severe congestive heart failure or valvular disease, were also excluded.
Studies published in languages other than the primary language of the review team (English), or missing translation resources, were omitted in order to reduce linguistic bias and maintain the feasibility and integrity of the review process. Furthermore, to avoid the inclusion of redundant data, duplicate studies or multiple publications of the same study were excluded.
Selection and Data Collection Process
Review selection
The selection process for this systematic review on the effectiveness of endovascular stenting in patients with SVCS followed a rigorous approach. A systematic approach was adopted to ensure dependability and accuracy whilst obtaining data. The reviewers independently screened all the titles and abstracts. Initially, a total of 117 studies were collected: 72 from Google Scholar, 25 from Cochrane, and 20 from Medline/Pubmed. The disagreements were resolved by discussion. Three duplicate studies were found and omitted. The process was repeated for full text article screening to determine the relevant studies, and as a result, 53 studies were excluded for lack of relevance. Two studies were not retrieved due to data silos. The remaining 59 studies were assessed for eligibility, and 50 of them were excluded because they did not adhere to the inclusion and exclusion criteria.
Data extraction
Three independent reviewers used a standardised protocol for data extraction from each study. The data included:
- Study design, sample size, patient demographic data (sex and age of patients)
- Procedural techniques used to treat patients with SVCS
- Reasonable data collection methods and medical reports of patient outcomes post-operatively
- Long-term follow-up outcomes
Any discrepancies among reviewers were resolved through discussion and consultation.
Outcomes
Primary outcome
The primary outcome of this systematic review was to comprehensively assess the efficacy of endovascular revascularisation of SVCS (technical and clinical success rate). Technical success of revascularisation was defined as suggested by the Society of Interventional Radiology (SIR): complete coverage of the lesion (stent overlapping the margins of the stenosis by 1 cm on either side) and residual stenosis of <30% as assessed by visual estimate. Clinical success was defined as relief of symptoms 48 hours after the procedure.7
Secondary outcome
The secondary outcomes included possible complications of endovascular stenting for SVCS. These complications include recurrence, headaches, confusion, seizures, or coma. Additionally, oedema of the larynx and upper respiratory tract may concomitantly occur, leading to difficulty in swallowing, voice hoarseness, and, potentially, a compromised airway. Further reported complications often arising in patients with SVCS include altered respiration, which leads to breathing difficulties, pleural effusion,8 and mortality.
Preparation for synthesis
In synthesising the data, a narrative summary of the included studies was created and presented in a table, emphasising the success rate after 48 hours, and common clinical complications in patients with SVCS.
Tabulation and Graphical Methods
Clinical trials found were grouped and tabulated according to year and category. For the production of efficient data analysis in a concise manner in this systematic review, clinical trials were presented in tabular format.
The data collected from all studies were collated and structured within a summary table. This table played a crucial role in categorising the data, forming the foundation for the results of the systematic review of all eight studies included in the review. To ensure transparency and adherence to inclusion and exclusion criteria, a PRISMA flow chart was created.
Methods to Explore Heterogeneity
The presence of heterogeneity in the systematic review was examined by analysing data collected through tabulation. The findings identified three to four distinct types of clinical trials. Despite this variation, the results of endovascular revascularisation in patients with SVCS were consistent across all trial types.
Assessment of Bias Risk
To reduce bias and enhance the reliability of the systematic review, stringent inclusion and exclusion criteria were used, with a particular emphasis on clinical trials. Furthermore, specific tools for assessing the likelihood of bias in systematic reviews, such as the one given by the Agency for Healthcare Research and Quality (AHRQ), were utilised.9 This technique allowed the reviewers of this systematic review to rigorously examine and address potential sources of bias in the included papers, improving the overall quality and validity of this review.
Reporting bias
To prevent and minimise the potential for bias, predefined criteria outlined in the methodology section were adhered to. Furthermore, findings were supported with robust statistical evaluation to provide objective evidence. Any observed discrepancies were explicitly acknowledged and addressed in both the results and discussion sections of this systematic review.
RESULTS
In conducting this systematic review, the authors focused on the investigative methods used to treat those with SVCS and the complications that follow. The utilisation of internet-based search tools proved instrumental in identifying a total of eight studies that shared a common focal point, presented in Table 1. This comprehensive analysis aimed to explore and evaluate the various approaches employed in managing this specific cohort of patients. By harnessing the power of online resources, this review gathered a diverse range of studies, allowing for a comprehensive synthesis of findings and evidence-based insights into the treatment strategies for SVCS.
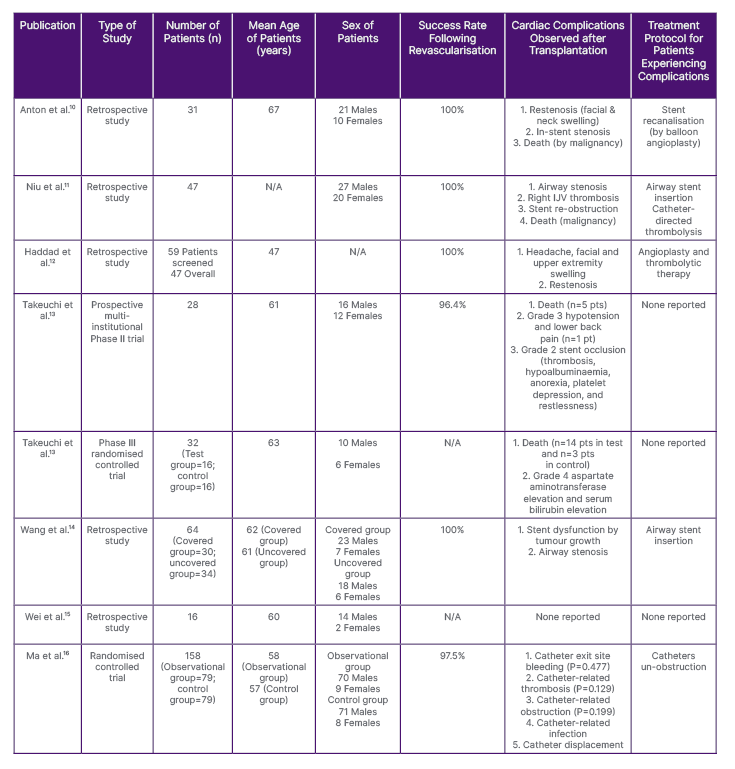
Table 1: Variables identified across the eight studies collated for the analysis for this systematic review on the success rate of endovascular revascularisation and complications arising in patients with superior vena cava syndrome.
IJV: internal jugular vein; N/A: not applicable; pt: patient.
Number of Patients Assessed
Across the eight studies listed within this systematic review, a diverse number of subjects volunteered to participate in each. In the study by Takeuchi et al.,13 32 patients were incorporated into the Phase III clinical trial, of whom 16 were categorised into a ‘control’ group and the remaining 16 into a ‘test’ group. The RCT conducted by Ma et al.16 consisted of 158 patients, similarly to the study by Takeuchi et al.,13 and the subjects were divided into two subgroups: 79 were placed into an ‘observation’ group and the remainder into a ‘control’ group. The treatment of malignant SVC blockage, which was the main priority of the retrospective study conducted by Wang et al.,14 showed that the patients within this study were divided accordingly; however, they were additionally categorised by the type of cardiac treatment being utilised within the study. For instance, 30 patients received a covered stent and 34 patients received an uncovered stent.15 Furthermore, initial screening of patients for potential access to stent treatment in the study by Haddad et al.12 resulted in 59 patients being selected overall. With more vigorous screening, 49 of the patients were selected following a secondary clinical diagnosis and a follow-up consultation.16
Technical Methods Utilised for Revascularisation
Optimal outcomes were achieved in the study by Wang et al.,14 in which airway insertion was achieved using either a covered stent (e.g., Fluency™, Becton, Dickinson and Company [BD], Franklin Lakes, New Jersey, USA) or an uncovered stent (e.g., E-Luminexx™, BD), both of which are highly efficient tools exceeding the overall diameter of the SVC, and which are essential for the treatment of SVCS. Similar cardiac instruments were chosen within the retrospective study conducted by Haddad et al.,12 in which the covered (e.g., GORE® VYBAHN®, Gore Medical, Newark, Delaware, USA; iCAST™, Getinge, Gothenburg, Sweden) and uncovered (e.g., WALLSTENT™, Boston Scientific, Marlborough, Massachusetts, USA; Protégé™, Medtronic, Watford, UK; S.M.A.R.T. ®, Cordis, Miami Lakes, Florida, USA) stents were used to treat the complications of SVCS. Next, according to the Haddad et al.12 clinical study, the balloon angioplasty procedure was effective in revamping specific types of stents (WALLSTENT, Protégé, and S.M.A.R.T.), which were used to correct any obstruction leading to SVCS. The majority of studies in this paper adopted the use of endovascular stenting as the procedure of choice to treat SVCS. Although, clinical femoral catheterisation was proven to be a more cost-effective and efficient, as well as accurate, technique to reach the targeted anatomical location (SVC), with reports of a low complication rate, specifically in the study by Ma et al.16
Revascularisation Success Rate
Among the various studies reviewed, the outcomes achieved through the utilisation of balloon angioplasty and endovascular stenting for the management of SVCS were favourable. Notably, in four of the eight studies, including those by Anton et al.10 and Niu et al.,11 a technical success rate of 100% was observed, signifying that all participants in these studies underwent successful initial treatment for SVCS. While the study conducted by Ma et al.16 reported a slightly lower success rate of 97.5%, in comparison with the perfect score of 100%, this figure still demonstrated a high degree of efficacy, indicating that a significant proportion of participants in that study were effectively treated using the endovascular stenting revascularisation technique. Unfortunately, data regarding the success rates of treatment in the Phase III trial by Takeuchi et al.13 and the retrospective study by Wei et al.15 were not obtainable.
Cardiac Complications Observed After Revascularisation
The reported complications observed in patients with SVCS after revascularisation varied across all studies. Since various techniques were implemented to treat patients with SVCS, complications cannot be attributed to endovascular revascularisation in general. Instead, the different treatment modalities are responsible for the diverse complications. In a study by Anton et al.,10 restenosis of the newly operated SVC was reported leading to secondary facial and neck swelling. Restenosis correspondingly was reported in the study conducted by Haddad et al.,12 where additional reported symptoms of headaches and upper extremity swelling were observed amongst patients. In many of the clinical studies selected for analysis, complications pertaining specifically to endovascular stent placement were reported. As exhibited in the two retrospective studies conducted by Niu et al.11 and Wang et al.,14 both provided reports of patients experiencing airway stenosis, secondary to endovascular stenting.
Furthermore, the study conducted by Ma et al.16 utilised a different surgical procedure than those reported in other literature; although complications pertaining to femoral catheterisation, such as catheter-related thrombosis, infection, and displacement were noted. Within three of the reported studies, death was a common complication arising amongst the reported population of patients. For instance, the Phase II trial and Phase III RCT conducted by Takeuchi et al.13 reported the deaths of five patients and 17 patients, respectively. Death was further reported in the study by Niu et al.;11 however, this was solely due to malignancy and not due to surgical treatment. Only one study out of the eight, that being by Wei et al.,15 failed to report any significant complications among patients after the procedure had begun to be used to treat the SVCS in its population of participants. All exhibited complications can be viewed below in Table 2.
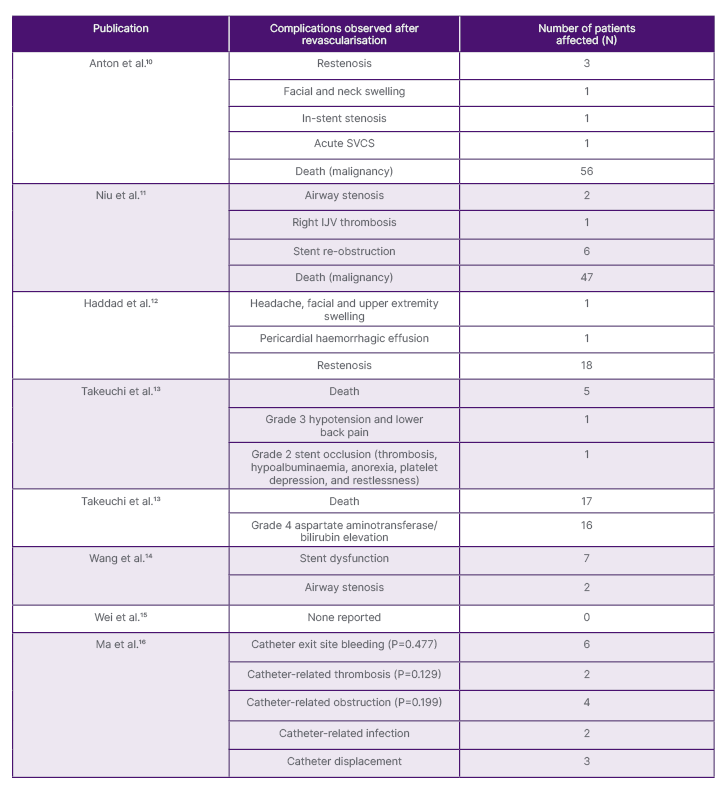
Table 2: Types of complications observed in patients with superior vena cava syndrome by data collation.
IJV: internal jugular vein; SVCS: superior vena cava syndrome.
Post-operative Treatment Protocol for Patients with Complications
Various treatment protocols were utilised by different studies to treat the cardiac-related complications that arose post-operatively in patients possessing SVCS. For instance, studies by Niu et al.11 and Wang et al.14 both similarly selected airway stent insertion to treat airway stenosis, secondary to endovascular stenting. Additionally, catheter-directed thrombolysis was proposed for patients partaking in the study by Niu et al.,11 a treatment regime replicated in a study of Haddad et al.12 Though the two studies shared similar post-operative treatment protocols, the study by Haddad et al.12 concomitantly offered angioplasty as a source of treatment for patients with SVCS experiencing complications. Treatment regimens specific for restenosis of the SVC were provided for patients in the study by Anton et al.10 through stent recanalisation and balloon angioplasty.12 Moreover, two of the studies conducted by Takeuchi et al.13 and the retrospective study carried out by Wei et al.15 did not report any significant treatment protocols for patients experiencing adverse complications from operative treatment administered for the correction of SVCS.
Follow-Up Period
The systematic review data reveal diverse patterns in the choice of post-procedural follow-up duration for patients. The Anton et al.10 retrospective study involved follow-ups for up to 184 days, whereas Niu et al.11 implemented a 6-month follow-up, leading to all patients’ unfortunate demise due to malignancy progression. In their retrospective study, Haddad et al.12 adopted a 3-month, 6-month, and yearly follow-up protocol, using the Kishi score >4 as a guide, along with digital subtraction venogram and contrast-enhanced CT venogram.17 Takeuchi et al.,13 in their prospective, multi-institutional Phase II trial, employed a 14-day follow-up by CTCAE score, continuing evaluation for 4 weeks. In their Phase III RCT, they utilised a 28-day follow-up after treatment, also using CTCAE score, and conducted weekly follow-ups with individual patients.13 Lastly, Wang et al.,14 in their retrospective study, employed a 14-month follow-up protocol, during which all patients succumbed to tumour progression or respiratory failure.
Prognosis for Patients With Superior Vena Cava Syndrome After the Procedure (Mortality, Survival Rate, Recurrence)
After undergoing repair of the impacted SVC, patients exhibited a diverse range of prognostic outcomes, as shown in Table 1. In both studies conducted by Takeuchi et al.,13 patients undergoing treatment had a calculated mortality rate of 17.9% and 25.0%. Regarding the median survival rate of all patients within the study by Anton et al.,10 patients treated by endovascular stenting had a median survival rate (MSR) of 180±248 days, while patients in the study by Niu et al.11 reported a similar MSR of 167 days.
Upon the comparison of both studies utilising the 2×2 factorial design method of grouping subjects for analysis, the study by Wang et al.14 reported an MSR of 175 days in the group of subjects receiving an uncovered stent, while those receiving a covered stent had a reported MSR of 159 days. Although a similar study design was utilised in the Phase III RCT by Takeuchi et al.,13 MSR of 67 days was reported in the test group, while the included control group reported an MSR of 93 days.13 Among all the studies, the retrospective study by Wei et al.15 had the largest documented number for MSR amongst its patients, with a total varying between 1–18 months. Only one out of the eight studies reported a proportion of patients experiencing recurrent episodes of SVCS, with 12.8% of all patients within the study by Niu et al.11 having recurrent episodes.
DISCUSSION
Age of Patients
Endovascular stent-based revascularisation was performed in patients with a mean age of 67±8 years in the study by Anton et al.,10 and patients with a mean age of 60 were utilised for a similar retrospective study conducted by Wei et al.15 Patients with a mean age of 58 years were in the observation group, and patients with a mean age of 57 years were in the control group in the Ma et al.16 randomised controlled clinical trial; whereas, in the Takeuchi et al.13 RCT, patients in the control group were of an average age of 57 years, and the patients in the observation group were of an average age of 63. Overall analyses of information acquired through web search engines suggest that the mean age of patients possessing SVCS, and who experience cardiac complications, lies around 59 years old. As malignancy tends to increase exponentially with age, the prevalence of SVCS among those over the age of 50 can be linked to malignancy, the main cause of SVCS. Thus, an increase in one’s age enhances one’s chance of developing SVCS.17
Sex of the Patients with Superior Vena Cava Syndrome
Despite the category of the study undertaken, there is a clear difference in the presented number of males and females studied within each clinical trial, with the population of males being significantly higher than the females. For example, the retrospective study by Anton et al.10 utilised 21 males and 10 females, while 27 males and 20 females were selected to partake in the retrospective study by Niu et al.11 Other studies exhibited a similar pattern of difference between the number of male and female participants included for review. For example, in the studies by Wang et al.14 and Wei et al.,15 more male participants diagnosed with SVCS were included in these studies, compared to female participants.11,15 Based on the available literature, SVCS is diagnosed when there is a disruption or obstruction in the transportation of blood through the SVC. This condition is considered a medical emergency and typically occurs in patients with a malignant disease process, primarily affecting the thorax.18 According to a study by Stabellini et al.,18 lung cancer is the leading cause of cancer-related deaths and the second most often diagnosed malignancy worldwide, with males having a higher incidence of lung cancer and a higher rate of mortality.19 Thus, there is a high probability that the elevated incidence rate of SVCS amongst male patients could be linked to the higher incidence of lung cancer seen in the male population. Generally, it was evident that more males were utilised in the selected studies used for this analysis of the use of endovascular stenting as a method of treating SVCS.
Methods Utilised for Revascularisation
Through analysis of all nine clinical studies conducted on the populous treatment type for patients diagnosed with SVCS, it was clear that endovascular stenting was the primary surgical technique employed as a form of first-line therapy. For example, in the studies by Anton et al.10 and Niu et al.,11 endovascular stenting was the primary form of treatment option utilised amongst the participants. This was due to supportive factors such as optimal procedural safety, lower cost, lower rate of complications, and better quality of life outcome that the surgical technique delivered.20 In the studies conducted by Haddad et al.12 and Wang et al.,14 covered stent use normalised all patients’ conditions, unlike uncovered stents. This was owed to the fact that covered stents had higher gross stent patency rates and lower stent occlusion rates than uncovered stents, thereby delivering a 100% technical success rate and 92% clinical success rate. However, the diameter of the covered stents used was kept 10–15% greater than the SVC diameter in order to prevent stent migration.21
Nonetheless, endovascular stent implantation was often required in patients who underwent balloon angioplasty, due to below par results and persistent stenosis outcomes. Moreover, SVC stent implantation provides a substantial 6-month remission from relapse of stenosis.22 Clinical femoral catheterisation was yet another surgical method that proved to be effective in revitalising patients with SVCS, according to the study by Ma et al.16 When conducted with the help of 2D-ultrasound guidance, puncture of the femoral vein, pseudoaneurysm formation, arteriovenous fistula formation, and further procedural complications were decreased by 49%, and first attempt success was increased by 42%.23
Types of Complications After Revascularisation
The adopted endovascular techniques outlined within this systematic review all had various levels of effectiveness, although complications arose with use. In the long-term follow-up trial conducted by Anton et al.,10 both endovascular stenting and balloon angioplasty were the preferred methods of treatment used on patients. However, associated complications, such as intra-stent thrombosis and death, were observed. Intra-stent thrombosis has been commonly recognised as a relative complication in approximately 16% of all patients post-stent deployment. The aetiology of intra-stent thrombosis can often be attributed to haemodynamic factors resulting from stent expansion within a vessel, as well as genetically classified or acquired hypercoagulability disorders in patients. These include antithrombin deficiency, protein S/C deficiency, or aplastic anaemia.24 Similarly, the use of endovascular stenting in the study conducted by Haddad et al.12 led to restenosis of the impacted SVC following surgery.10 Commonly, restenosis arises due to the pathophysiological process of hypertrophic wound healing, occurring as a regulatory mechanism to override vascular inflammation, which arises due to stent implantation and vascular injury.25
Studies by Niu et al.11 and Wang et al.14 both presented reports of airway stenosis as a secondary complication arising from the utilisation of endovascular stenting to treat persistent SVCS.14,15 With the use of tracheal intubation during surgical procedures, patients often have a high probability of developing airway stenosis as the overriding cuff pressure rises above that of the normal capillary pressure of the trachea, resulting in the ischaemia and long-term ulceration of the tracheal cartilages. This, in turn, induces fibrotic healing mechanisms that progressively damage the tracheal tube.26 Lasty, amongst several studies, including both clinical trials conducted by Takeuchi et al.,13 a small proportion of patients died as a result of cardiac transplantation treatment methods. Death can arise as a late complication following the treatment of SVCS, mainly as a consequence of anticoagulation. Thus, anticoagulant therapy by the use of warfarin, clopidogrel, or aspirin, is given as a source of prophylactic treatment to patients prior to surgery and following a given risk assessment.27
Prognosis for Patients With Superior Vena Cava Syndrome After Revascularisation
As observed acoss several studies highlighted in this systematic review, many of the patients treated with endovascular revascularisation either experienced adverse side effects, acute recurrences of SVCS , or, consequently, did not survive. For example, in the studies by Anton et al.10 and Niu et al.,11 a large proportion of patients died following treatment.10,14 All of the patients presented in these two studies were initially diagnosed with various forms of malignancy or mass effect, both of which cause shear stress on the venous intimal layer and damage to the SVC.28 Malignant causes of SVCS include oesophageal cancer, mediastinal tumours, and small-cell lung carcinoma, with the latter accounting for 50% of all diagnosed patient cases.13 Recurrent episodes of SVCS were observed in both studies by Niu et al.11 and Haddad et al.12 Episodes of SVCS arising amongst medically treated populations can be accounted for by several factors; for instance, the increased incidence of pacemakers, and their associated leads, used to treat alternate cardiovascular diseases such as those listed in the paper by Wei et al.15,27
Treatment Protocol for Patients with Superior Vena Cava Recurrence
From the studies sourced for this systematic review, Takeuchi et al.13 utilised endovascular techniques to treat patients with SVCS experiencing complications post-surgery; however, such were not specified within the paper.13 According to a paper by Ponti et al.,29 successful endovascular therapy ensues in more than 95% of patients, and over 90% of them report symptom relief.29 Further analyses state that within the study by Anton et al.,10 stent recanalisation (by balloon angioplasty) technique was chosen to fix specific complications experienced, including in-stent stenosis or restenosis of the SVC.10 In this regard, a report by Volpi et al.20 indicates that this technique does not interfere with subsequent antitumour treatments and provides urgent relief of symptoms, as well as additionally improving the quality of life in patients with benign causes of SVCS.20 In the studies by Niu et al.11 and Wang et al.,14 airway stent insertion was used to rectify all complications associated with airway stenosis following surgical treatment, though all patients died due to tumour progression or respiratory failure.10,15 Consequently, no definitive conclusion can safely be made on the authority of which treatment protocol is suitable for patients being treated for complications, secondary to the treatment of persistent SVCS.
CONCLUSION
Significant advancements have been observed in the developed therapeutic approaches for managing patients diagnosed with SVCS. However, further comprehensive research is imperative to enhance patient survival rates and mitigate morbidity arising from common complications subsequent to therapeutic intervention for this condition. Malignancy continues to emerge as the primary causative factor in the development of this medical disorder. Hence, the implementation of additional protocols, including screening measures and the utilisation of prevailing diagnostic modalities, is warranted to ensure timely identification and treatment of cancer, thereby reducing the predisposition to SVCS. Additionally, alternative endovascular approaches and anticoagulation therapy show promise in mitigating primary causes of SVCS, warranting further investigation to enhance outcomes for the general population.







