Meeting Summary
This symposium took place during the 2019 meeting of the European Association for the Study of Diabetes (EASD). Focussing on the kidney as a window to the heart, the speakers discussed connections between the kidney and the heart, potential mechanisms, and the role of sodium–glucose co-transporter 2 (SGLT2) inhibitors in patient management. Prof De Nicola set the scene with projected numbers of patients with diabetes and diabetic nephropathy. Prof DeFronzo gave a description of the natural history of diabetic nephropathy, microalbuminuria as a predictor of chronic kidney disease (CKD), and the Steno hypothesis linking impaired vascular endothelial dysfunction with vascular leakage of albumin. He concluded his talk by describing why patients with CKD are predisposed to cardiovascular disease (CVD). Prof Groop provided insights into the mechanisms of renal protection by SLGT2 inhibitors. He explained the ‘tubular hypothesis’, whereby SLGT2 inhibitors correct glomerular hypertension by inhibiting tubuloglomerular feedback (TGF). Prof Perkovic highlighted data from randomised controlled trials which enhanced understanding of the potential effects that might be achieved with SLGT2 inhibitors. The meeting concluded with a lively discussion between panel members and the audience.
Welcome and Objectives
Professor Luca De Nicola
The objectives of the meeting were to actively exchange scientific knowledge with the faculty and the audience on Type 2 diabetes mellitus and the potential role of SGLT2 inhibitors in reducing CV and renal events. The number of patients on dialysis is expected to double in the next few years, exceeding 5 million by 2030.1 Data from the Global Burden of Disease study suggests that >275 million people had renal disease in 2016.2 Diabetes is the primary reason why patients need renal replacement therapy,3 and approximately half of all patients with Type 2 diabetes mellitus worldwide also have diabetic kidney disease.4 Existing therapeutic tools for diabetic nephropathy are insufficient. Renin–angiotensin–aldosterone system (RAAS) inhibition alone only reduces the risk of end-stage renal disease by up to 25%.5,6
The Kidney as a Window to the Heart
Professor Ralph DeFronzo
Early in the natural history of diabetic nephropathy, there were few clinical or laboratory abnormalities that identified patients at risk of developing clinically overt diabetic kidney disease.7,8 Patients with diabetes may appear to have good kidney function and even a normal kidney biopsy. Despite this, the kidney is not perfectly normal. A study of individuals with a strong family history of diabetes who had a kidney biopsy showed evidence of hyperfiltration 3 years before diabetes was diagnosed.9
In the first 15 years after diabetes onset, there is a clinically silent period until proteinuria develops. Kidney biopsies reveal continuing hyperfiltration and glomerular sclerosis. Once macroproteinuria develops, progression to end-stage renal disease is relatively inevitable. Within 4 years azotaemia develops, and 3–4 years later patients require dialysis or transplantation; however, there is another clue that indicates the need for earlier intervention: the development of microalbuminuria. This typically occurs about 5 years before the onset of macroproteinuria and effective intervention may slow, and possibly prevent, overt disease.7
Multiple studies have documented that Type 1 diabetes mellitus patients with microalbuminuria have a 10–20-fold increased likelihood of developing advanced renal disease within 10 years.10-13 Although not as strong a predictor as in Type 1 diabetes mellitus, microalbuminuria is also linked with a 4–5-fold increased risk of diabetic nephropathy in patients with Type 2 diabetes mellitus.14 Microalbuminuria predicts overall survival in patients with Type 2 diabetes mellitus. Over a 9.5-year period, survival relative to the general population reduces as the severity of microalbuminuria in Type 2 diabetes mellitus patients rises.14 The dramatic fall in survival is primarily due to an increase in CVD.
People with microalbuminuria have marked widespread endothelial dysfunction, primarily due to lack of nitric oxide which is a powerful vasodilator and antiangiogenic molecule. Microalbuminuria also tracks with dyslipidaemia, hypertension, left ventricular (LV) diastolic dysfunction (the characteristic cardiac dysfunction in patients with diabetes), and plasminogen activator inhibitor-1 levels which predisposes to hypercoagulability increasing CV risk. Microalbuminuria is part of the insulin resistance or metabolic syndrome, which encompasses all of the risk factors associated with accelerated CVD.15,16 These include obesity, prediabetes, diabetes, hypertension, dyslipidaemia, increased plasminogen activator inhibitor-1, endothelial dysfunction, lipotoxicity, liver disease, inflammation, atherosclerotic CVD, and hyperinsulinaemia. As it can be considered that uraemia (glomerular filtration rate [GFR] <25 mL/min/ 1.73 m2) is a state of insulin resistance, patients with advanced renal disease can be said to have metabolic syndrome.16-18 Numerous prospective epidemiologic studies have established that insulin resistance and the insulin resistance syndrome predict CVD and future diabetes. People with insulin resistance have a 2.5–3-fold higher likelihood of a CV event over the next 10 years.
The Steno hypothesis proposed that albuminuria results from widespread vascular damage.19,20 Microalbuminuria reflects impaired vascular function and is associated with susceptibility to both CV and renal events. This hypothesis links impaired vascular endothelial function with vascular leakage of albumin detected in the urine; thus, it can be said that the kidney becomes a window to the vasculature with ‘leaky’ renal vessels reflecting the widespread permeability of the vasculature. Albuminuria predicts the risk of myocardial infarction, stroke, heart failure, and death in people with and without diabetes.21,22 Studies also show that CKD is related to an elevated likelihood of CV events and death.23 CV event rates progressively increase with declining GFR.24
Patients with CKD are predisposed to CVD because the two conditions share the traditional risk factors for atherosclerosis.25 Patients at high-risk tend to be older, males, smokers, and physically inactive. Comorbidities such as hypertension, dyslipidaemia, diabetes/insulin resistance, LV hypertrophy, and a prothrombotic state also increase risk. Additional risk factors related to CKD include endothelial dysfunction, vascular calcification, uraemic toxins that trigger inflammation, increased reactive oxygen species and oxidative stress, excess extracellular fluid, and glomerular hyperfiltration. Renal hyperfiltration is also a strong predictor of CV events.26 Potential mechanisms for the relationship between renal hyperfiltration and CVD include endothelial dysfunction, increased renin–angiotensin system activity, increased Na+/H3 pump, hyperglycaemia and insulin resistance, oxidative stress, inflammation, fibrosis, and hypertension.
In summary, patients with CKD are at markedly increased risk for CVD. Risk factors for CVD in patients with CKD include both classic and additional risk factors. Microalbuminuria is a major risk factor for future kidney disease and atherosclerotic CVD. If renal vessels are damaged, this is a marker of damaged vessels throughout the body; thus, the kidney serves as a window to the health of the heart and arterial system.
The Interlinking of Cardiovascular and Renal Disease in Type 2 Diabetes
Professor Per-Henrik Groop
Mortality is higher in patients with CKD and Type 2 diabetes mellitus than in people without diabetes.27 With the development of albuminuria or reduced estimated GFR (eGFR), or both, the risk of premature mortality is many times higher. Diabetic kidney disease comes with real consequences, and this is mainly due to CVD.28 As kidney function declines and more albumin leaks into the urine the risk of CV death increases. Impaired renal function has far-reaching systemic effects, which include insulin resistance,29 arterial calcification, anaemia, hypertension, inflammation, LV hypertrophy,30 sympathetic nervous system activation, RAAS activation,31 and endothelial dysfunction.32
A recent meta-analysis of the exploratory secondary renal endpoints of worsening, end-stage renal disease or renal death in the SGLT2 inhibitor CV outcomes trials suggested clinically important effects on these endpoints both in patients with established CVD and in those with multiple risk factors.33 The question arises as to why do SGLT2 inhibitors work so well in the kidneys? Looking at renal physiology, the kidney autoregulates the flow of blood through the glomerulus by altering arteriole tone.34 The tone of the afferent arteriole decreases by a number of mechanisms: nitrogen oxide bioavailability, COX-2 prostaglandins, kallikrein-kinins, atrial natriuretic peptide, angiotensins I-VII, hyperinsulinaemia, and inhibition of TGF (Figure 1).35 The macula densa works as the control tower of the glomerulus and plays a major role in the autoregulation of glomerular blood flow. Factors that cause a net increase of efferent arteriolar pressure are angiotensin II (one of the most potent vasoconstrictors), thromboxane A2, endothelin 1, and reactive oxygen species. This balance between the afferent and efferent arterioles by autoregulation is very important for the function of the kidney.
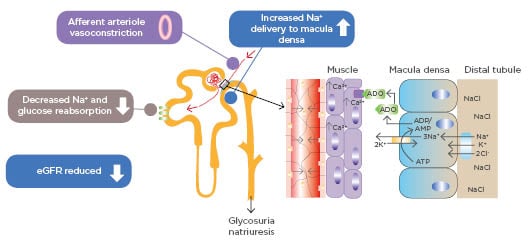
Figure 1: Sodium–glucose transport protein 2 inhibition and tubuloglomerular feedback.
ADO: adenosine; ADP: adenosine diphosphate; AMP: adenosine monophosphate; ATP: adenosine triphosphate; eGFR: estimated glomerular filtrate rate; SGLT2: sodium–glucose co-transporter.
Adapted from Heerspink HJ et al.35
In situations where there is an imbalance, the intraglomerular pressure can increase with the development of glomerular hypertension. This causes glomerular damage and subsequent progressive nephron loss.36 The remaining nephrons adapt, but by further increasing filtration via glomerular hypertension.36 SGLT2 inhibitors appear to improve renal outcomes by reducing pathological intraglomerular pressure and may consequently slow nephron loss.35,36
A leading theory to explain how SLGT2 inhibitors act to prevent kidney decline is the so-called ‘tubular hypothesis’.35 With large amounts of glucose being filtered, the kidney upregulates the SLGT2 receptors to preserve glucose; consequently, more glucose is reabsorbed from the tubules along with co-transported sodium. This has the effect of less sodium reaching the macula densa. This is interpreted physiologically as a drop in glomerular filtration pressure. Inappropriate autoregulation by the macula densa causes an increase in both the filtration and blood pressure within the glomerulus. SLGT2 inhibitors correct this abnormal situation by blocking glucose and sodium absorption in the proximal tubule.
More sodium ultimately reaches the macula densa, and via autoregulation, the afferent arteriole contracts thereby reducing overall glomerular pressure.
There are numerous studies supporting this hypothesis. SGLT2 blockade reduces afferent artery diameter and single nephron GFR (SNGFR) in diabetic mice.37 In diabetic rats, acute SGLT2 blockade reduced SNGFR by 33% compared to control (p<0.0005), while chronic SGLT2 blockade reduced SNGFR by 16% versus control (p<0.03).38 There was a significant difference between acute and chronic blockade.38
In patients with Type 1 diabetes mellitus, angiotensin-converting enzyme (ACE) inhibitors reduce glomerular hyperfiltration by opening up the ‘back door’ of the glomerulus (the efferent arteriole)39 and SGLT2 inhibition reduces glomerular hyperfiltration to a similar extent, but act by closing the ‘front door’ (the afferent arteriole).40 The effects of SGLT2 inhibition on renal blood flow and vascular resistance mediate the reduction in hyperfiltration.40 There is also evidence that SGLT2 inhibition and RAAS blockade may have synergistic effects since their effects are mediated on afferent and efferent arterioles, respectively.41 In both the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program42 and (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),43 the effect of SGLT2 inhibition on renal markers occurred irrespective of the use of an ACE inhibitor or angiotensin receptor blocker. In patients with Type 2 diabetes mellitus and impaired renal function, SGLT2 inhibition also reduces blood pressure and glycated haemoglobin (HbA1c).44 Other studies show that SLGT2 inhibitors increase albuminuria regression compared with placebo.45,46 SGLT2 inhibition also increases urinary ketone bodies.47
An additional reason for the effectiveness of SLGT2 inhibitors is that they may spare the kidneys from hypoxia. When diabetes is induced in mice, within 3 days the kidneys become relatively hypoxic.48 In rats, hypoxia (induced by uncoupling the mitochondria) causes kidney disease independently of hyperglycaemia and oxidative stress.49 The phenotype of that induced hypoxia is proteinuria or albuminuria, inflammation, and cell damage, as seen in diabetic kidney disease. In patients, reduced cortical oxygenation predicts progression of renal decline.50 Data also show that SGLT2 inhibition improves mitochondrial function.47
Hyperfiltration in diabetes increases sodium handling in the proximal tubule51 and 90% of oxygen consumption in the kidneys is due to sodium handling by the proximal tubule.52 Increased sodium handling increases oxygen consumption and so raises the risk of renal hypoxia and CKD.51 SGLT2 inhibitors prevent this hypoxia53 without inducing acute kidney injury.45,54,55
In summary, SGLT2 inhibitors protect the kidney through loss of calories due to glucosuria leading to modest weight loss,35 osmotic diuresis and modest natriuresis,35 reduction in the development or worsening of albuminuria,35,56 reduced glomerular hyperfiltration via TGF feedback,35 and possible synergistic effects with hypertensive agents.57
The Role of the Sodium–Glucose Transport Protein 2 is Changing in Cardiovascular and Renal Outcomes
Professor Vlado Perkovic
Approximately half of all patients with Type 2 diabetes mellitus have concomitant diabetic kidney disease;4 thus, renal outcomes and their effects on CVD are highly relevant. Over the last half century, although there has been a two-thirds reduction in deaths due to CVD in the USA, the number of people with kidney failure has dramatically increased.58,59 Diabetes is the leading driver of kidney failure and better therapies are needed.
The only proven treatment for renal disease to date is RAAS blockade. Angiotensin receptor blockers and ACE inhibitors produce a 23–28% reduction in end-stage renal disease (Figure 2),5,6 with a suggestion that these drugs delay kidney failure by 6–12 months, a relatively modest impact on these clinically important outcomes.
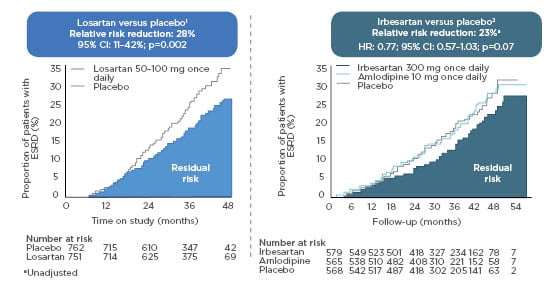
Figure 2: Renin–angiotensin system inhibition alone does not reduce the risk of end-stage renal disease adequately.
N.B., trials shown had different designs and patients populations and cannot be directly compared.
CI: confidence interval; ESRD: end-stage renal disease; HR: hazard ratio; RAAS: renin–angiotensin–aldosterone system.
Adapted from Brenner BM et al.5
In patients who received irbesartan in The Irbesartan Diabetic Nephropathy Trial (IDNT) or losartan in the Reduction of Endpoints in NIDDM [non-insulin-dependent diabetes mellitus] with the Angiotensin II Antagonist Losartan (RENAAL) Study, the proportion who developed kidney failure was still large: one-quarter over 4 years in RENAAL and a similar proportion in IDNT. It is clear that new treatments are required to curb the rising numbers of patients with kidney failure around the world.
Since 2008, regulatory agencies have required CV outcome trials for new diabetes drugs. These studies have also shed light on their effects on the kidney through exploratory renal endpoints. In the four CV safety trials of dipeptidyl peptidase-4 (DPP4) inhibitors (SAVOR-TIMI 53,60,61 TECOS,62 EXAMINE,63 and CARMELINA),64 no strong signal of renal benefit was observed. In the CARMELINA trial, for example, the secondary renal composite outcome was not reduced with linagliptin, even though it lowered HbA1c by about 0.35%.64
For the glucagon-like peptide 1 receptor agonists, a meta-analysis of CV outcome trials shows an overall 17% reduction in risk of the composite kidney outcome (hazard ratio [HR]: 0.83; 95% confidence interval [CI]: 0.78–0.89).65 Most trials, however, included new-onset macroalbuminuria as a component of that outcome. If macroalbuminuria is removed and the focus is only on major kidney outcomes i.e., doubling of serum creatinine, or substantial losses of kidney function, the collective HR is 0.87 (95% CI: 0.73–1.03). This suggests a modest overall benefit for the glucagon-like peptide 1 receptor agonists, despite quite profound effects on HbA1c.
The SGLT2 inhibitor CV safety trials had secondary renal composite outcomes of worsening renal function, end-stage renal disease, and renal death. The findings suggested a benefit, even though those trials that did not include patients at high renal risk.33 When the results are broken down by baseline eGFR (>90, 60–90, and <60 mL/min/1.73 m2) there is evidence of benefit in all groups (HR: 0.44; 95% CI: 0.32–0.59, HR: 0.56; 95% CI: 0.46–0.70, HR: 0.67; 95% CI: 0.51–0.89, respectively). There is an indication, however, of declining kidney function attenuation (p=0.0258). There were relatively few patients with an eGFR <60 mL/min/1.73 m2; so, it remains unclear whether the benefit of SGLT2 inhibitors persists down to lower levels of kidney function.
Additional limitations of these CV safety trials are that the renal outcomes, when prespecified, were secondary exploratory endpoints; definitions of renal endpoints differed; baseline characteristics of the study population varied, and the follow-up period was short (2.4–4.2 years).43,45,55,66 This is reflected in the small number of hard renal endpoints in these trials. Despite recruiting >34,000 patients and completing almost 100,000 years of patient follow-up in total, 54 patients, across all 3 trials, developed end-stage kidney disease and 69 progressed to renal replacement therapy or experienced renal death. While these trials support a benefit, they are not sufficient to definitively demonstrate the renal benefits of SGLT2 inhibitors.
With regards to CV events in these patients, a meta-analysis of the three CV safety trials suggested that protection against heart failure is greatest in patients with reduced kidney function, a 40% reduction in risk (p=0.0073).38 Consistent findings for heart failure were reported in the renal outcomes trial;30,69 similarly, there is a clear overall benefit of SGLT2 inhibitors on major CV events, particularly in patients with an eGFR <60 ml/min/1.73 m2.33,67,68
What is the price to be paid for this ‘triple whammy’ of benefit on kidney failure, heart failure, and major CV events? A renal outcomes trial with canagliflozin showed an increase in the risk of genital mycotic infections in men and women, which were generally easily treatable and did not require discontinuation of treatment, and a raised likelihood of diabetic ketoacidosis.56 There was no increased risk of urinary tract infection, volume depletion adverse events, acute kidney injury, fracture, or lower extremity amputation.56
The latter result differed from CANVAS Program, where an elevated risk of lower extremity amputation was observed.69 Following the signal in CANVAS, a protocol amendment was introduced into the renal outcomes trial recommending closer foot care. This amendment did not impact recruitment to the trial and there was no change in the relationship between study drug and lower extremity amputation before and after the protocol was amended. In CANVAS this was not a prespecified outcome whereas in the renal outcomes trial it was prospectively tested, raising the possibility that the finding in CANVAS was a chance observation.
A recent meta-analysis of the CV safety trials and renal outcomes trial indicates a benefit of SGLT2 inhibition on substantial loss of kidney function, end-stage kidney disease, or renal death, even in patients with a baseline eGFR <45 ml/min/1.73 m2.66 The analysis suggested some attenuation of relative risk reduction in patients with the lowest baseline eGFR (p value for heterogeneity=0.073), although the absolute benefits were at least as large in this subgroup.66 When the results were examined according to baseline albuminuria, there were broadly consistent benefits across all trials, even in patients with microalbuminuria or normal values.66 Across the 4 trials, there was also an overall 25% reduction in acute kidney injury with SGLT2 inhibitors (relative risk: 0.75; 95% CI: 0.66–0.85).66
Other SGLT2 inhibitor renal outcomes trials with dapagliflozin and empagliflozin are ongoing, these will define whether the kidney benefits are a class effect or not, and provide further insights into patients with and without diabetes, and varying levels of baseline eGFR and albuminuria.70,71
Standards of care are already changing. The American Diabetes Association (ADA) guidelines have a Grade A recommendation for the use of SGLT2 inhibitors in patients with Type 2 diabetes mellitus and kidney disease to prevent both CKD progression and CV events.72 The 2019 European Society of Cardiology (ESC) guidelines have also recommended SGLT2 inhibitors in patients with CKD and Type 2 diabetes mellitus.73 One issue for clinicians following the guidelines in treating CKD with the SGLT2 inhibitor class is that current labelling does not allow their use in patients with eGFR <45 ml/min/1.73 m2. This may change as the regulators review the emerging data from this class.
Interactive Panel Discussion
Professor Luca De Nicola, Professor Ralph DeFronzo, Professor Per-Henrik Groop, Professor Vlado Perkovic
The session concluded with a lively and lengthy discussion among panel members and the audience. Topics included the causes of diabetic nephropathy, how best to monitor patients on SGLT2 inhibitors, the need for concomitant treatments, and how to improve implementation of this new class of drugs.








