Abstract
This clinical treatment modality was applied to 62 diabetic ulcers on lower extremities for which surgeons had been advised amputation. Total healing was achieved in 53 of them and was achieved through this treatment in a 3-month period. The remaining nine cases also showed improvement in healing at different levels, but they were not accepted as a ‘satisfactory result’. This treatment modality contains a synthetic prostacyclin analogue, two different phosphodiesterase inhibitors, a peripheral revascularisation agent, another agent increasing peripheral resistance to ischaemia, and a polysaccharide with positive rheologic properties on capillary circulation. Therefore, this treatment was found to be effective on circulation of the extremities, with radiologically-proven insufficient blood supply. The treatment also had a positive effect on recirculation and effects on collateral revascularisation through mechanical vacuum application, modified from standard vacuum treatments. With this combination, this technique was found extremely effective by application, according to the algorithm explained below, and should be an alternative to the current therapy applications in diabetic ulcers.
Key Points
1. Diabetic foot wounds are usually a result of diabetes-related vascular disease and neuropathy, and present with various symptoms such as inflammation, foot lesions, and a purulent discharge.2. This article explores how diabetic foot wound develop and provides case studies on treated patients, including insight into the challenge of treating heavy smokers.
3. The author discusses the possibility of preventing diabetic foot wound through patient education and supportive treatments.
INTRODUCTION
Although there are many reasons for the development of diabetic foot wound, the main reason is the combined effect of diabetes-related vascular disease and neuropathy. Aerobic Gram-positive cocci are mostly responsible for superficial diabetic foot infections (DFI), which develop in patients with cellulitis who have not used antibiotics before. Pseudomonas aeruginosa is one of the common causes when the patient’s toes remain wet.1 After excluding other causes, DFI should be considered when at least two of the classic signs of inflammation are present, including redness, warmth, swelling, tenderness, pain in the foot lesion, or presence of purulent discharge.2
Patients diagnosed with DFIs are primarily classified as mild, moderate, or severe in terms of severity of infection, based on criteria such as the depth and width of the wound, and whether there are systemic signs of infection.1-3 The perfusion, extent, depth, infection, and sensation system should be the preferred classification system as it has a high predictive value for diabetes-related foot complications.3
A culture sample from a diabetic foot wound should be obtained only when clinical infection is suspected and before starting antibiotic therapy if possible. Biomarkers such as leukocyte count, C-reactive protein (CRP), erythrocyte sedimentation rate, and procalcitonine, which are indicators of inflammation, may be useful in distinguishing between infection and colonisation.4,5 MRI is a sensitive and specific method for patients who cannot be mobilised, and who are thought to have osteomyelitis or deep soft tissue abscess, to assess the response to treatment. The gold standard in the diagnosis of osteomyelitis is histopathological examination. Requirements to ensure wound healing and to save the leg are the removal of dead and infected tissues with urgent and aggressive debridements, appropriate antibiotic therapy, metabolic control, relieving the foot from weight and pressure, diagnosis and appropriate treatment of peripheral arterial disease, and regaining the function of the foot. The fact that the factors playing a role in its ethiopathogenesis are very different, complicates the developing lesions and requires a team understanding in the approaches to such patients.6,7
Diabetic foot problems are the only complications of diabetes that can be prevented through education.6
Major Mechanisms Involved in Diabetic Wound Development
The major mechanisms involved in diabetic wound development are nerve hypoxia/ischaemia; collagen molecules disorders (elastin, proteoglycan); auto-oxidative stress; endothelial dysfunction; increased activity in the polyol pathway; mitochondrial dysfunction; increased advanced glycation products; lack of growth factors; γ-linolenic acid deficiency; changes in the immune mechanism; increase in protein kinase C, especially B isoform; increase in protease secretion; dysfunction of cytokines; and others. All of these factors should be interpreted separately and in combination, to achieve a satisfactory result in DFIs.8-14
Local and Systemic Treatment of Diabetic Foot Ulcer
Wound care is one of the local and systemic treatments of diabetic foot ulcer. In wounds with risky circulation and sensory protection, this care should feature aggressive debridement of necrotic tissues; local chemical (antiseptic) and antimicrobial (antibiotic) treatment for infected tissues (systemic antibiotic in wound culture positive with single pathogen cases only); vacuum-combined dressing (different from standart vacuuming, where the negative pressure is applied continuously and up to -600 mmHg, and wound coverage is obtained through a mixture of nitrofurazone ointment and rifamycine applied gauze with its specific colour, sensitive to pH changes and covered with transparent film dressings; the colour changes from orange to white and indicates the wound dressing change periods); balancing dressing techniques according to secretion status (wet to dry, dry to wet); observing the wound with transparent covering material without changing the dressing; obtaining data from the distal part of the lesion by pulse oximetry if possible; and measurement of extremity temperature at room temperature from the distal of the lesion, which is another parameter for wound healing.
Further treatment should include the treatment of circulatory failure and neuropathic component, with rheological agents such as low molecular weight (LMW) dextran (MW:40), pentoxifylline, trimetazidine, cilostazole, synthetic prostacyclin, and tadalafil.1-5
Low Molecular Weight Dextran
Investigations in this new field received great impetus from the discovery and commercial availability of dextrans. These polysaccharides have the ability to alter blood-flow properties. The alterations vary with the molecular weight of the dextran. LMW dextran has demonstrated its ability to decrease blood viscosity and reverse cell aggregation, producing an increase in blood flow and tissue perfusion. It has been suggested that the value of LMW dextran will best be determined by observations on its effectiveness in relieving symptoms and diminishing complications, in conditions known to cause stasis in blood vessels.15
Pentoxifylline
Effect Mechanism
As a phosphodiesterase inhibitor, it prevents cyclic adenosine monophosphate degradation, and increases glycolysis and endogenous adenosine triphosphate production. It also increases erythrocyte flexibility and has an antiaggregant effect (with a dextran- like mechanism).
Therapeutic indications
Therapeutic indications include occlusive diseases of peripheral arteries and arteriovenous circulation disorders caused by arteriosclerotic or diabetic causes (such as intermittent claudication, rest pain); trophic disorders (such as leg ulcers and gangrene); cerebral circulation disorders; and circulatory disorders of the eye and ear with a degenerative vascular process.16
Trimetazidine
Effect Mechanism
Trimetazidine prevents the decrease in intracellular adenosine triphosphate by preserving energy metabolism in cells exposed to hypoxia or ischaemia. In this way, it ensures the proper functioning of the ion pumps and the transmembranal sodium-potassium flow. Trimetazidine increases glucose oxidation by blocking long chain 3-ketoacyl-CoA thiolase and inhibiting β-oxidation of fatty acids. In an ischaemic cell, the energy obtained during glucose oxidation requires less oxygen consumption than the β-oxidation process. Enhancement of glucose oxidation optimises cellular energy processes, and thus maintains proper energy metabolism during ischaemia.17
Cilostazole
This the first choice drug, as 3–6 months of evidence shows an increase in both treadmill exercise performance and quality of life. It is an effective drug to improve symptoms and increase walking distance in patients with lower extremity peripheral artery disease and intermittent claudication. It is recommended in all patients with life-limiting claudication.18
Synthetic Prostacyclin
Iloprost (ZK36374) has a cytoprotective effect, as it kept the kidney tissue alive for 7 days in vitro. It also increases tolerance to ischaemia in the heart muscle and striated muscle. It is a peripheral potent vasodilator and antiaggregant, and is the major component of the treatment.19
Tadalafil
This medication is added to the treatment later for the expected rheologic improvements, following the publications explaining positive effects on microcirculation by vasodilation. It was applied on the last 23 cases and was well tolerated, especially by male patients, according to the rest of the treatment components.20
METHODOLOGY
The treatment begins with pentoxyfilline 900 mg solutions applied with LMW dextrane 500 mL daily and 50 mL on an hourly basis. Synthetic prostacycline is also applied (20 µg) on an hourly basis via a different intraveinous route, following hospitalisation and monitoring of cardiac functions and vital signs. This intraveinous treatment is limited to 12 hours a day, and for rest of the day only the additional fluid requirements are provided if necessary. At the same time trimetazidine 80 mg and cilostazole 100 mg per day (on the seventh day cilostazole dose increased to 200 mg daily) are administered orally. Finally, tadalafil 5 mg daily is added to this polipharmacy group.
The side effects mainly belong to pentoxfilline and syntetic prostacycline, and include nausea, vomiting (which is easily controlled by antiemetics), flushing on face and body, hypotension, and slight tachycardia transiently in the first 5 days of treatment. Blood sugar alterations were frequent due to rheologic agents (mainly LMW dextrane), and close monitoring and proper treatments were applied to regulate blood sugar levels. In most of the cases, management was effective, and this has been checked by lowering HbA1c levels in the 6-week period following the treatment. Treatment continues for 10 days as a combination, mainly in the hospital. Following the first treatment period, the patient is discharged from the hospital with oral pentoxyfilline, trimetazidine, cilostazole, and tadalafil in the same doses. The follow-up is continued for 12 weeks on an outpatient basis, unless a surgical step to cover the wound (such as a skin grafting or local flap procedures) is necessary.
White blood cell and procalcitonine levels were not accepted as important statistically (with student T-test) because of the results’ stability independently from the clinical status of the patients. CRP and erythrocyte sedimentation rate (ESR) levels seemed important as the initial period (15–30 days) of treatment eliminated the infectious agents from the wound, reflecting the parameters with the absence of a serious finding and circulation restoration. The parameters of the extremity circulation improvement were; temperature, colour, capillary filling (if present, the refilling time following application of finger pressure over the skin), and measurable oxygen saturation at the distal part of the extremities. Blood sugar levels and HbA1c levels were also important because of the effectivity of the treatments’ indirect effects on blood sugar variations.
Patients in this study had HbA1c levels of minimum 8.5 and maximum 10.9 (mean: 9.7) and were aged between 48–84 years. There were 39 males and 24 females. Nine of 62 patients were accepted as ‘unsatisfactory results’. Of those patients, eight had a limiting factor such as heavy smoking (more than a package per day), and six of them were male. The last patient was a female who smoked and had systemic lupus disease. These cases were all subject to amputation, and four amputations were performed. The other five refused the amputation and follow-ups are still continuing, without a significant improvement in the treatment of their ulcers.
RESULTS AND DISCUSSION
Complete recovery was achieved with this method in 53 out of 62 cases with indication for amputation, and this state of well-being continued for at least 2 years.
The longest follow-up of cases was 11 years; however, in six cases (four of whom were smokers), patients required additional treatment sessions starting from the second year, and these sessions yielded successful results in all of them. None of them required another session of treatment although their risk factors were still present. The theoretical explanation could be the neovascularisation on a capillary level (Figures 1–3). There was no control group except for six patients who refused the treatment. All of them underwent an amputation in the following 2 years. Some parameters for diabetes and wound healing were observed and checked by laboratory findings, during and following the treatment. Most of them showed statistically important improvements and indicated the effectiveness of this treatment.
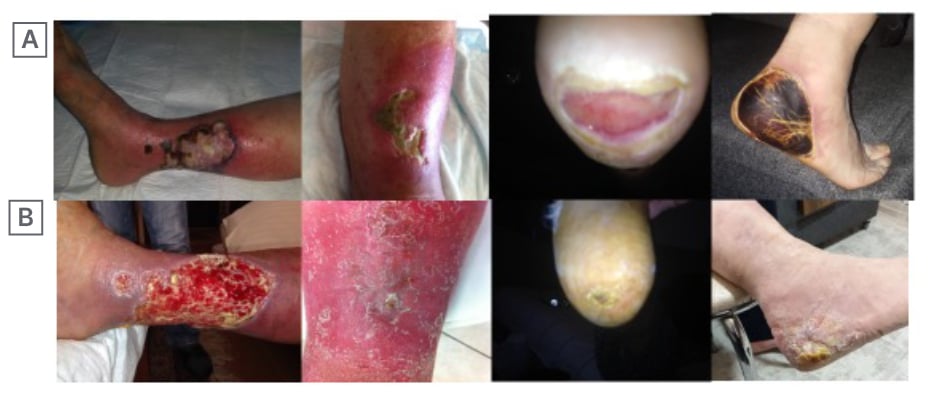
Figure 1: Pre-treatment of four cases (A) and appearance of the cases following treatment (B) in a 3-month period.
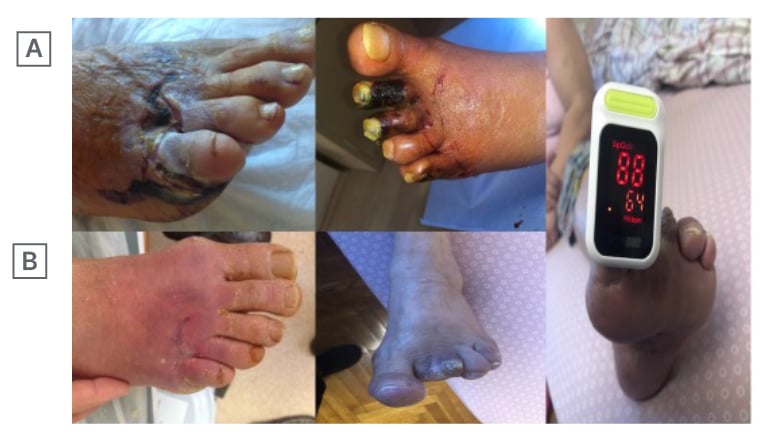
Figure 2: These cases had very high levels of HbA1c (such as 10.9) and after only 15 days of treatment (including amputation of necrotic parts), the ulcers were completely ephitelialised and survived against high levels of HbA1c for 4 years.
A) Pre-treatment and B) post-treatment with measurable oxygen saturation (which was impossible at the beginning).
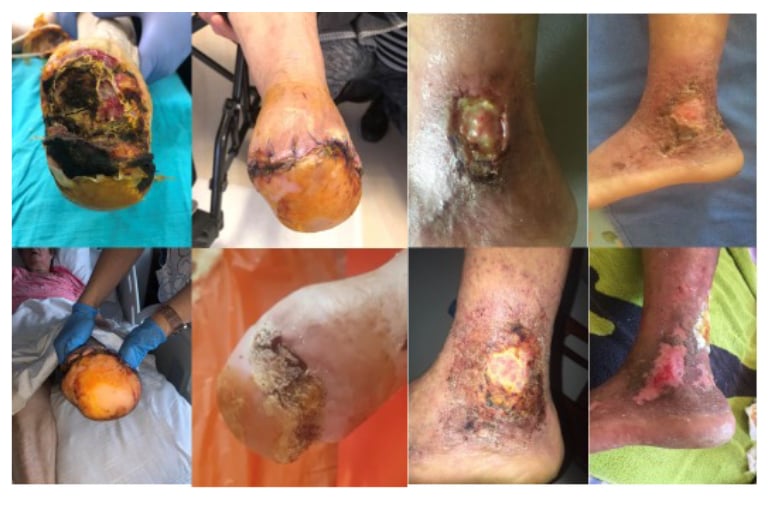
Figure 3: These two cases were resistant to treatment in the first 8 weeks because of pre-existing smoking, and continuing another 4 weeks resulted in healing.
Cessation of smoking gives better results following reduction of blood nicotine levels to normal.
In the diabetic foot, it is possible to prevent complications with supportive treatment and patient education. For the foot ulcers with poor control of blood sugar, this modality is effective not only for wound healing, but also for the control of the blood sugar levels in the post-healing period.6 The HbA1c levels showed an approximately 15% decrease in the first 6 weeks and up to 30% in a 12-week period with this treatment in 52 cases. This decrease is also accepted as an indicator for the effectiveness of this modality. As a control group, the rest of the 62 patients (nine cases) also showed some improvement on their wounds and blood sugar levels, but 12 weeks of treatment did not result in ulcer healing. In 52 of the cases, the CRP levels were higher than normal (approximately 25%) for the whole population. Following the ephitelisation, in a 3-week period, 46 patients showed a return to normal levels of CRP and the rest of the cases remained above the normal levels of CRP. In these cases, Hba1c levels did not alter more than 10% and autologous wound coverages were not more than 20% of the wound surfaces (Table 1). The dimensions of the wounds were calculated by milimetric paper measurement as a classic parameter in wound healing research. Finally, four of the cases were amputated below knee level, and five of patients did not allow amputation and continued with wound dressing changes, without further complications. Therefore, even for the failure of the treatment cases, there is still effectiveness in the later periods, and up to 2 years there was no progression on wounds, or further complications such as gangrene or osteomyelitis.
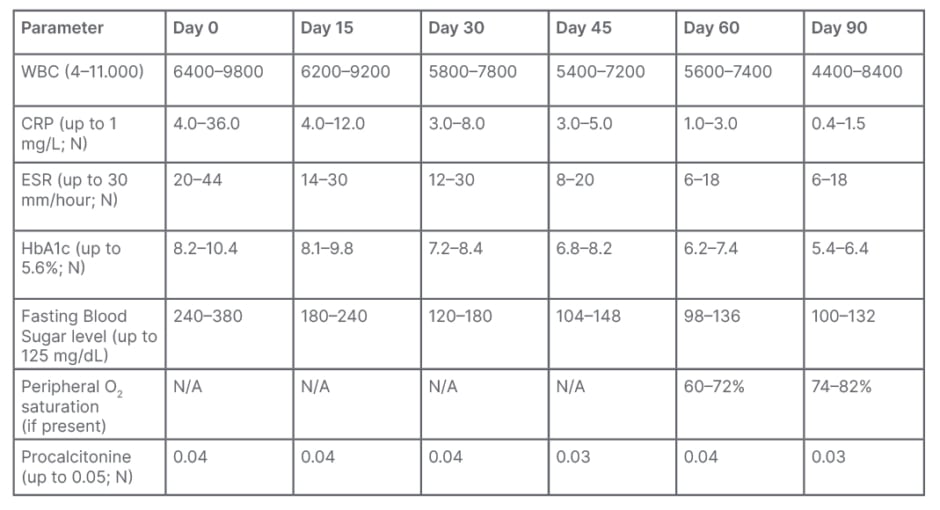
Table 1: Clinical and laboratory parameters for follow-up of the patients.
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; N/A; not applicable; WBC: white blood cell.
In addition to the treatment, new pharmaceuticals (glucagon-like peptide, glifozine, and their variations) have been put forward for the regulation of blood sugar, which have positive effects on the continuity of the treatment (Figures 1–3). It can be said that there is now a serious treatment alternative for the diabetic foot ulcers with this method, even when there is an indication for amputation.21
CONCLUSION
In conclusion, this treatment modality seems extremely effective in an area with too many ineffective methods described in literature; however, it is not a solution for the treatment of all types of diabetic ulcers. There are some restrictions and limitations of the treatment such as a history of myocardial infarction in last 6 months, presence of heavy smoking or another pre-existing vascular disease, and inability to control the blood sugar levels. Therefore, the number of 53 out of 62 cases with complete healing and without recurrence in at least 2 years on diabetic ulcers proves a treatment alternative on this unsolved problem. As a result, it can be said that, with a careful verification of the limiting and restricting factors, this method should be a first alternative in the therapeutic arsenal of the treatment of diabetic ulcers.







