Abstract
Introduction: Hybrid closed loop (HCL) systems have the potential to improve glycaemic control in people with Type 1 diabetes (T1D). In France, patient technical education and assistance for HCL users is provided by trained nurses from home healthcare providers (HHP). The objective of this study was to evaluate satisfaction of people with T1D with HHP services.
Methods: In total, 35 participants with T1D and a prescription for an HCL system were studied during 3 months after HCL initiation in two French hospitals. A series of questionnaires were completed by participants. The number of planned (per protocol) and unplanned HHP interactions was monitored. Glycaemic control at inclusion and Day 90 was compared; formal statistical testing was carried out post-hoc.
Results: Client Satisfaction Questionnaire (CSQ-8) with HHP service was high both at Day 30 (mean CSQ-8 score: 28.9; 95% confidence interval [CI]: 28.0; 29.9) and at Day 90 (29.0; 95% CI: 27.9; 30.0). Hypoglycemia Fear Survey-II (HFS-II) score (standard deviation) decreased from 31.2 (±15.7) at inclusion to 23.1 (±16.8) at Day 90.
Participants had a median number of four home visits and two phone calls, but important differences were observed between participants: total interactions with HHP nurses ranged between five and 12 contacts, and 45.7% of participants requested unplanned interactions.
Glycaemic control improved significantly: mean time in range increased from 57.0% (±13.3) at inclusion to 71.4% (±9.4) at Day 90 (p<0.001).
Conclusion: HHP services for early phase HCL implementation were met with high client satisfaction levels. Study results emphasise the need for a personalised HHP approach.
INTRODUCTION
HCL systems have the potential to improve glycaemic control in people living with T1D.1-4 HCLs combine continuous glucose monitoring (CGM) and algorithm-directed insulin pump delivery, such that glucose levels are continuously controlled and corrected.5 Despite increased use of insulin pumps and CGM devices, glycaemic targets are often not achieved among adults with T1D. Approximately 20% of patients achieve HbA1c target levels.6,7
The benefits of HCL systems were confirmed in observational studies in real-life settings.8-11 Time in range (TIR; blood glucose: 70–180 mg/dL) was 70–80% in HCL users, and increased by approximately 10% compared with pre-HCL values.8-11 HCL may increase independence, alleviate the burden of insulin management and glucose monitoring, reduce the risk of hypoglycaemia, and improve quality of life.2,9,12
Patients with T1D who start using an HCL device will need technical and educational assistance to become proficient in the safe handling of the HCL system and its components. Assistance is especially important in the early phase of using an HCL system. The key to successful use of HCL devices lies in patient motivation, knowledge, and building trust in the system.13 Lack of either element may lead to early discontinuation or reduced health benefit.14
In France, nurses from HHPs have a specific role in the management of patients using insulin pumps, CGM devices, and, more recently, HCL systems. HCL setup and installation are carried out at hospital specialised centres, with knowledge and skills in intensive diabetes management with assistance from HHP nurses. Once discharged from hospital, HHP nurses allow for a smooth transition from hospital to home. They supply devices to patients’ home, participate in their installation, replace equipment when necessary, provide initial and continuous technical training, register patients on the remote monitoring platform, and support and ensure information exchange between patients and clinicians.15-17
HHPs may be of particular value during the first few months to reduce the risk of early discontinuation. The need for HHP support may gradually decrease as patients get more proficient in the use of their HCL system.13 There is little information about the optimal organisation in providing initial support to patients initiating on an HCL in France, and globally.
The primary objective of this study was to evaluate patient satisfaction with the HHP service before, during, and after installation of an HCL system under real-world conditions. Additional objectives were to assess hypoglycaemia-related constraints, knowledge acquisition, number of HHP interactions, glycaemic outcomes, and safety.
![]()
In France, ‘prestataire de santé à domicile’ (HHP) is an integral part of the French healthcare system. The first HHP services in France appeared in the 1980s to provide O2 therapy to patients at home, thereby avoiding hospitalisation. Since then, the range of services has been continuously extended to cover other therapeutic domains, including sleep apnoea, respiratory insufficiency, and insulin-therapy. Today, HHP nurses provide technical assistance and equipment to approximately 3.5 million patients in France each year.18 HHP nurses receive training in both the specific therapeutic area by expert clinicians and on the technical aspects of the HCL system by the device manufacturer. HHP personnel constitute an important link between patients, their treating physician, and medical care centres/hospitals through constant communication and feedback. Interactions with HHP nurses can also take place at patients’ homes, providing additional comfort, especially for people with reduced mobility and in rural areas. HHP services are reimbursed by the French healthcare system.
![]()
METHODS
This was a multicentre, longitudinal, non-comparative, interventional study in people with T1D initiating on an HCL system (SATURN, NCT04635280).19 The study was conducted in two hospitals in France. HHP nurses for this study were part of Air Liquide subsidiaries (Air Liquide, Paris, France). The study was approved by an independent ethics committee and carried out in respect of good clinical practice guidelines, the Declaration of Helsinki, and French law.
Participants aged ≥18 years were eligible if they had a history of T1D of at least 2 years, and used an insulin pump for at least 6 months. HbA1c levels had to be <10% during the previous 4 months. The participating centres pre-selected patients with T1D based on their medical file. Patients who met the inclusion/exclusion criteria were invited to participate in the study and underwent the baseline visit to confirm eligibility. All participants received the t:slim X2™ HCL pump (Tandem Diabetes Care, San Diego, USA) with integrated Control-IQ® technology (Tandem Diabetes Care), associated with the Dexcom G6 CGM (Dexcom, San Diego, USA).
The study was divided into pre-installation, installation, and 3-month follow-up phases. The main goals of the HHP service were to provide training for the HCL system, ascertain proper functioning and completeness of the equipment, supply and replace equipment, provide technical on call assistance, and liaise with clinicians throughout the study. The number of visits and contacts were adapted based on participants’ needs, as well as investigator and HHP staff judgement. Investigators were responsible for verifying inclusion/exclusion criteria, informed consent, initiating the HCL system, biological tests, evaluating the patient’s metabolic parameters transmitted via the online monitoring platform, decision regarding continuation of HCL system, and collection of adverse events.
The primary endpoint of this study was participants’ satisfaction with the HHP service as determined via the CSQ-8.20,21 Additional endpoints included the HFS-II score,22 knowledge acquisition evaluated through a tailor-made questionnaire, the number of contacts between HHP nurses and participants, and glycaemic control (HbA1c and glucose metrics obtained by CGM). The different questionnaires used in this study are described below.
Client Satisfaction Questionnaire
The CSQ-8 is a validated questionnaire, which has been translated to several languages and applied to a range of healthcare settings, including childbirth and mental health.23
The CSQ-8 consists of eight questions, each of which has four possible answers. The questions assess the quality of the service, whether the service corresponds to participants’ expectations, whether needs are met, and problems solved. Participants rate overall satisfaction and whether they would recommend the service to a friend or use the same service again. The maximum total CSQ-8 score is 32, with higher scores indicating greater client satisfaction.
Hypoglycemia Fear Survey-II
Hypoglycaemia can be a life-threatening event. The constant fear of experiencing hypoglycaemic events can be stressful to patients with T1D, and reduce their quality of life.24 To avoid such events, patients may restrict social interactions, keep higher glucose levels, avoid activity, and recur to frequent blood sugar measurements.
The HFS-II is a validated questionnaire, translated into several languages, consisting of two subscales for behaviour (HFS-B) and worries (HFS-W). The HFS-B consists of 15 questions, each with five possible answers, with focus on specific behaviours to avoid hypoglycaemia. Typical items are ‘limit physical activity’, ‘eat large snacks’, or ‘keep blood glucose >15 mmol/L’. The HFS-W assesses the worries related to hypoglycaemic events and includes 18 questions (each with five possible answers); for example ‘not realising having low blood sugar’, ‘low blood sugar interfering with important things’, or ‘becoming hypoglycaemic during sleep’. Responses add up to subscales and total scores with maxima of 60 (HFS-B), 72 (HFS-W), and 132 (total HFS-II). Higher scores indicate that patients are more affected by hypoglycaemia events.
Knowledge Acquisition
The knowledge acquisition questionnaire is a tailor-made tool that was developed by HHPs, not validated psychometrically, to evaluate patients’ knowledge regarding HCL use over time. The questionnaire is divided into basic and advanced functions, and comprises 15 categories; for example: ‘knowledge on the use of the pump’, ‘knowledge on the control module menu’, ‘knowledge on the precautions for use of the components’, or ‘programming specific boluses’. The HHP assesses the knowledge acquisition for all items in each category using a 4-point Likert scale (‘not addressed’, ‘not acquired’, ‘in progress’, and ‘acquired’).
This questionnaire allows HHPs to follow their patients’ progress and to deliver patient-centred training, which focuses on those areas where patients present knowledge gaps.
In this study, knowledge was considered acquired if the response to a specific item was either ‘acquired’ or ‘in progress’. The percentage of participants with knowledge acquired at installation, Day 30, and Day 90 was summarised using descriptive statistics.
Participants completed the CSQ-8 and the HFS-II at the beginning of the study, and 1 and 3 months after HCL installation. Participant satisfaction was considered achieved if the CSQ-8 score at Day 30 was ≥20 points and the CSQ-8 score at Day 90 was ≥20 points and not decreasing more than four points with respect to the Day 30 evaluation. HFS-II scores, knowledge acquisition, number of HHP contacts, and glycaemic outcomes were described using descriptive statistics. Post hoc analyses using formal statistical comparison between glycaemic parameters at inclusion and Day 90 were carried out, using a paired t-test or McNemar test. A sample size of 32 participants was considered sufficient to estimate the percentage of participants satisfied at Day 90 with a precision of ≤12.4%. All analyses were carried out with the software package SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) or higher.
RESULTS
In total, 35 people with T1D were enrolled between May–September 2021, including 20 at site one and 15 at site two. All participants completed the study. Mean age was 42.4 (±11.5) years, and 21 (60%) participants were male. The mean time since T1D diagnosis was 23.3 (±12.4) years, ranging between 4–61 years. Of these participants, 24 (68.6%) had at least one comorbidity at inclusion; the most common comorbidities were metabolism and nutrition disorders, reported in 14 (40%) participants. At inclusion, 14 (40%) participants had at least one diabetes-related complication.
All participants had prior use of an insulin pump and CGM device, including intermittently scanned CGMs. The mean time since first installation of insulin pump was 10.9 (±6.9) years. Seven (20%) participants had a history of HCL system use.
Primary Endpoint: Client Satisfaction Questionnaire
The overall CSQ-8 score was high. At Day 30, mean CSQ-8 score was 28.9 (95% CI: 28.0; 29.9), and at Day 90 it was 29.0 (95% CI: 27.9; 30.0; Table 1). The mean change in CSQ-8 score between Day 30 and Day 90 was 0.1 (95% CI: -0.8; 0.9), suggesting that satisfaction levels were maintained during the study period.
All participants rated the quality of HHP service as either ‘good’ or ‘excellent’. All participants were ‘mostly’ or ‘very satisfied’ with the help they received and HHP nurses were able to deal with problems (‘somewhat’ or ‘a great deal’) according to participants’ perception.
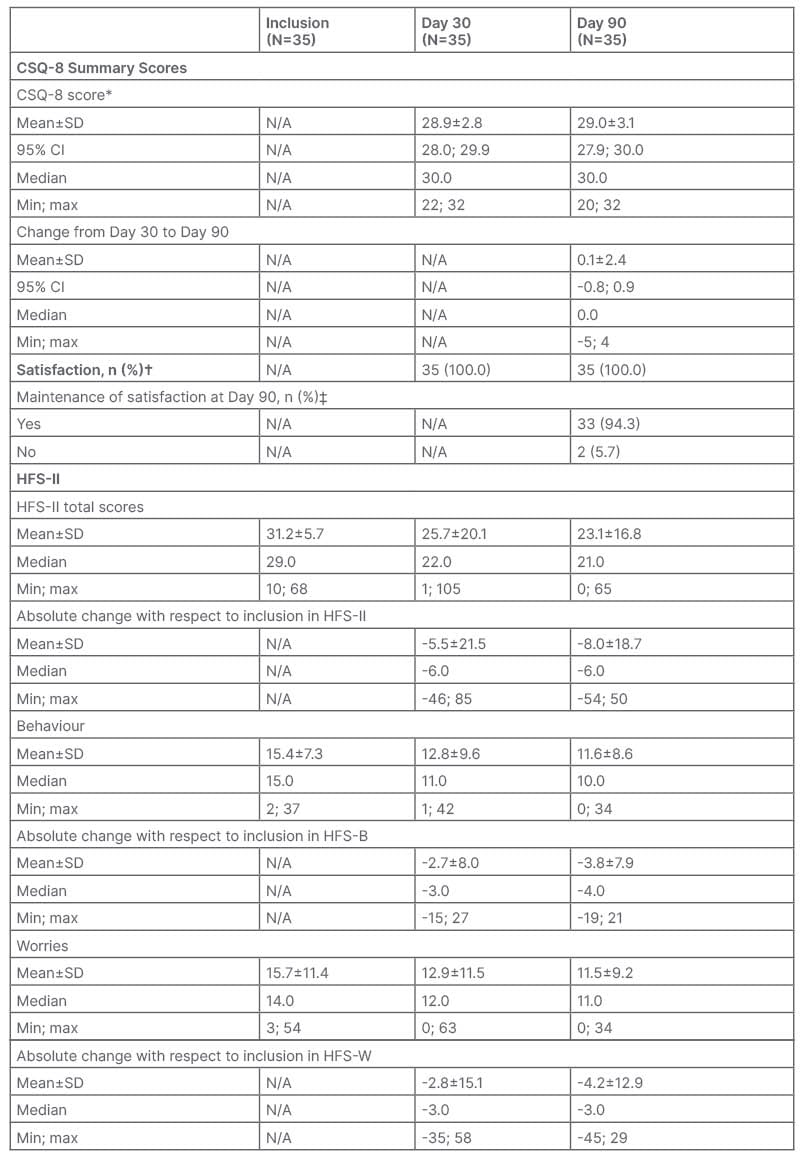
Table 1: Overview of Client Satisfaction Questionnaire and Hypoglycemia Fear Survey results.
*Score ranges from 8–32, with higher values indicating higher satisfaction.
†Score ≥20.
‡Score at Day 90 ≥20 that did not decrease by more than four points compared with Day 30.
CI: confidence interval; CSQ-8: Client Satisfaction Questionnaire; HFS: Hypoglycemia Fear Survey; HFS
B: Hypoglycemia Fear Survey – Behavior; HFS-W: Hypoglycemia Fear Survey – Worries; max: maximum; min; minimum; N/A: not applicable; SD: standard deviation.
Hypoglycemia Fear Survey
The overall mean (standard deviation) HFS-II score decreased from 31.2 (±15.7) at inclusion to 23.1 (±16.8) at Day 90 (Table 1). A decrease in scores was seen in both the behaviour and worries subscales. The mean HFS-B score decreased from 15.4 (±7.3) at inclusion to 11.6 (±8.6) at Day 90. The mean HFS-W score decreased from 15.7 (±11.4) at inclusion to 11.5 (±9.2) at Day 90.
Home Healthcare Provider Interactions
HHP interactions were planned at specific time points per protocol, namely during HCL installation, early follow-up, at Day 30, and Day 90. All other visits were considered as unplanned.
Most participants had four home visits and two phone calls, but important differences were observed between participants; total HHP interactions ranged between five and 12 contacts. Overall, there were 7.3 (±1.5) HHP interactions on average, resulting in a mean cumulative time of approximately 8 hours during the 3-month study period. Approximately half of participants (45.7%) required additional unplanned contacts, with the majority being at the patient’s request. Reasons for these unplanned interactions were additional training needs, device-related incidents, sensor installation support, support for data upload, replenishment needs, management of alarms, motivational support, or need of reassurance.
Knowledge Acquisition
At the early follow-up visit (Day 1/3), 74.3% of participants had acquired the knowledge provided during their initial training. Areas that needed to be revisited most often, i.e., knowledge not yet acquired in ≥4 participants at Day 1/3, included re-sugaring, sensor start-up, start-up in closed loop mode, status bar and icons on the terminal, difference between self-calibration and calibration mode, and safety mode. Participants still in the learning process had achieved 94.1% (±3.6%) of required knowledge at Day 1/3. At Day 90, all but two participants had fully acquired the required knowledge, while the remaining participants achieved 98.4% of required knowledge.
Glycaemic Control
Glycaemic parameters consistently improved during the study period (Table 2). Mean HbA1c value was 7.4% (±0.8%) versus 6.7% (±0.6%) at inclusion and Day 90, respectively (p<0.001). The proportion of participants achieving HbA1c values <7% increased from 28.6% at inclusion to 62.9% at Day 90. At the same time, the proportion of participants with an HbA1c ≥7 and <8 decreased from 51.4% to 37.1% while no participant had HbA1c values ≥8 at Day 90 compared with 20% at inclusion.
Mean time spent in glucose range, defined as ≥70 to <180 mg/dL, was 57.0% (±13.3%) at inclusion and 71.4% (±9.4%) at Day 90 (p<0.001). Mean percentage time spent below range (<70 mg/dL) decreased from 4.7% (±4.6%) at inclusion to 2.1% (±1.7%) at Day 90 (p<0.001) and mean percentage time above range (>180 mg/dL) decreased from 37.8% (±14.9%) at inclusion to 26.4% (±9.4%) at Day 90 (p<0.001).
All participants continued their treatment by HCL after the end of the study.
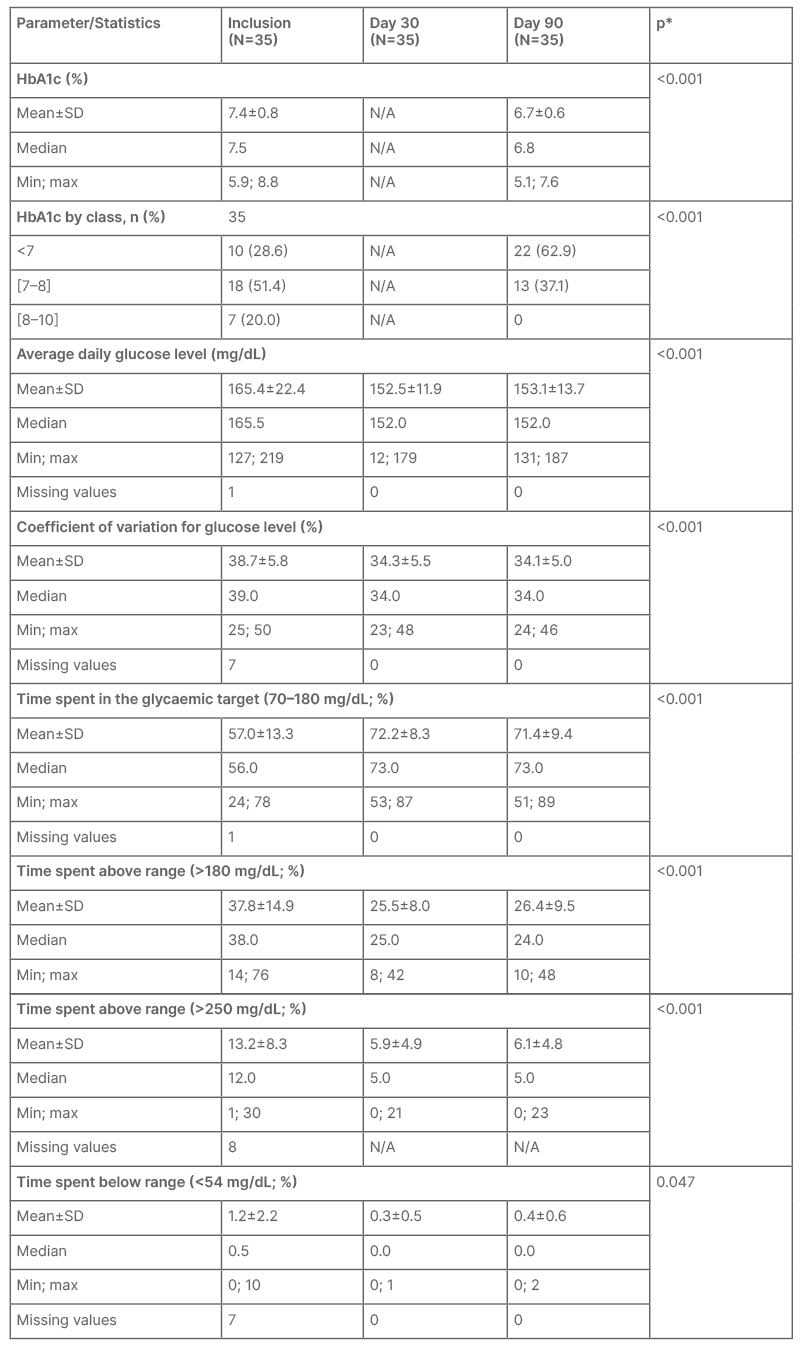
Table 2: Metabolic control at inclusion, Day 30, and Day 90 (intention-to-treat population).
*Paired t-test or McNemar test to compare values at Day 90 to values at inclusion.
Max: maximum; min; minimum; N/A: not applicable; SD: standard deviation.
Safety
Twenty participants experienced a total of 38 adverse events (AE), either mild or moderate. There were no serious AEs. Three participants (8.6%) had a total of four events of hyperglycaemia with ketosis, and 12 AEs were related to the device, mainly hyperglycaemia and/or ketosis and catheter site-related AEs. Overall, 25 (71.4%) participants reported a total of 55 incidents with the HCL system, mainly related to catheter issues. No severe hypoglycaemia events were reported.
DISCUSSION
In this study, participants with a prescription for an HCL system received educational and technical support from HHP nurses for 3 months. The authors assessed participants’ satisfaction with the HHP service using a validated questionnaire: the CSQ-8. The overall satisfaction level was high and constant over the study period. Most participants responded that they were satisfied with the quality of the service and the help received.
The goal of the HHP service is to ensure that patients acquire the knowledge and proficiency required to use the HCL system, and to develop trust in the system and its algorithm. Trust and knowledge are paramount in order for patients to ‘let go’ and ‘hand over control’ to the system.
HHPs also provide motivational support and reassurance such that patients may draw full benefit of their HCL system, e.g., improved glycaemic control and fewer diabetes-related concerns, such as hypoglycaemic events. Qualitative research with clinical study participants receiving an HCL device highlighted that participants had ambivalent feelings at the beginning of the study.13 Building trust and confidence in the HCL system required several weeks during which participants scrutinised the algorithm and the functioning of the HCL device. Once they understood the workings and realised that the device made sensible adjustments, they were able to step back and let the system do its job. Participants emphasised the benefit of tailored training and the opportunity to ask in-depth questions in the early phases of HCL use. Also, memorising all features at once is illusionary and certain functionalities will only be acquired over time, making additional training sessions essential.
Results from the authors’ study agree with qualitative research and emphasise the need for a personalised service. Not all participants had acquired the necessary knowledge at the early follow-up visit, but the percentage of knowledge acquisition increased with subsequent HHP interactions. There were variations in the number of planned and unplanned interactions, highlighting the need for a personalised approach. A patient-centred service may provide ad hoc assistance, reduce the risk of early HCL discontinuation, and improve the health benefits of HCL devices. This echoes the viewpoint of clinicians who highlight the importance of high-quality training to new HCL users both in clinical research and real-life setting.14 In a longitudinal study in children and young adults with T1D, 28 out of 91 participants (30%) discontinued HCL within 6 months.25 Major hurdles and reasons for discontinuations were ‘technical issues/problems’, ‘too much work to maintain HCL’, ‘expense/reimbursement’, and ‘alarms’.25 Thus, technical assistance is equally important for trouble shooting and resolving technical issues that otherwise could lead to frustration.
Metabolic balance improved during the study with an increase in TIR of 10 percentage points, which is considered clinically meaningful. TIR is strongly associated with microvascular complications; a decrease in TIR by 10 percentage points was shown to increase the probability of developing retinopathies by 64% and that of kidney disease by 40%.26 No severe hypoglycaemia events were reported during the study period.
There are several limitations to this study: the research was conceived as a feasibility study to evaluate satisfaction with HHP services before the introduction of this new technology on the market. Therefore, the sample size was relatively small, and the study duration was limited to 3 months. In this study, all participants continued to use the HCL device after study completion, but larger studies with a longer follow-up period are needed to determine the discontinuation rate of HCL users who receive assistance from HHP services. Patients with HbA1c ≥10% were excluded in this study. Patients with poorly controlled glycaemia may benefit most from the HCL system. The value of HHP services in this patient group deserves further attention, especially in light of higher HCL discontinuation rates observed among patients with higher HbA1c values at baseline.25 The HHP service is a patient-centred approach with the objective of ensuring patient satisfaction, lowering discontinuation rates, and improving health benefits, including quality of life. Such objectives are consistent with the notion of value-based healthcare, i.e., optimising health outcomes from a patient perspective while minimising costs to the healthcare system.27
No comparator arm was included in this study as there was no ‘standard of care’ for HCL assistance in France at the time of the study. Therefore, it is not possible quantify the contribution of HHP services to the observed improvements in glycaemic control and HFS-II values. Technical assistance for insulin pumps is historically provided by HHP services in France, and this service could be naturally extended to HCL systems. All participants received the same HCL system in this study because only one HCL system with Conformité Européenne (CE) mark was available during the recruitment period; however, HHP nurses are trained on different models and their assistance is not specific to a single model. The French HHP model may alleviate the burden on healthcare professionals who are often under time pressure, by delivering educational and technical assistance to patients with an HCL system.28
CONCLUSIONS
The HHP service as implemented in this study reached high satisfaction levels among participants. HHP service represents an important step towards a value-based healthcare system, providing patient-centred and personalised support. Improvements in glycaemic control and HFS-II scores need to be viewed in the context of study limitations, i.e., small sample size and short follow-up period. Studies with longer duration are needed to determine discontinuation rates and estimate the contribution of HHP services to optimise health benefits and quality of life of patients, while lowering the burden to the healthcare system.







