Abstract
Background: Recent studies have revealed conflicting results for low glycaemic index (GI) meals in the prevention and treatment of metabolic disorders. Diurnal variations in glucose homeostasis, which are frequently overlooked in epidemiological studies, may help to explain some of these discrepancies.
Food is an external cue to entrain the circadian rhythm, and meal timing is a crucial factor for glucose homeostasis. The study examines the relationship between chrono-nutrition, chronotype, and blood glucose control among people with Type 2 diabetes.
Materials and Methods: Chrono-nutrition questionnaire assessed eating window, breakfast skipping, evening latency, evening eating, night eating, and largest meal of patients with Type 2 diabetes. Glycaemic control was assessed using a HbA1c test, fasting blood sugar, and 2-hour post-prandial blood sugar test. Insulin resistance was assessed by fasting triglyceride glucose index (TyG).
Results: There was a significant association between late dining with dysglycaemia, irrespective of GI of the meal (p<0.05). Participants who had the largest meal during the active phase had better glycaemic control (p<0.05).
Shorter eating windows and evening latency of at least 2 hours not only aided in glycaemic control, but also gave good sleep (p<0.05). Participants with the evening chronotype ate almost twice the amount of carbohydrates and fat at dinner than at breakfast. Evening chronotypes were associated with lesser servings of vegetables and fruits, and greater servings of sweets and caffeinated beverages, in comparison with morning chronotypes.
Conclusions: Late diners had significantly worse blood glucose levels, irrespective of the GI of the meal. This may have public health implications, as calorie-dense meals are often consumed during late evenings, which can desynchronise the circadian rhythms. Eating meals as per the circadian rhythm could be an alternative non-pharmacological strategy to prevent diabetes and its complications.
Key Points
1. Chrono-nutrition is an emerging field, studying the interaction between temporal dietary patterns, circadian rhythm of our body, and metabolic health.
2. Behavioural indicators of the chrono-nutrition profile, such as a shorter eating window, no breakfast skipping, long evening latency, early dinner, and eating the largest meal early positively affect metabolic health.
3. Early low-GI dinners had a better glycaemic response than late low-GI dinners.
INTRODUCTION
Type 2 diabetes (T2D) is a persistent metabolic anarchy, described by hyperglycaemia resulting from defects in insulin secretion, insulin action, or in a combination of both.1 T2D, once assumed to be a metabolic condition only seen in affluent Western countries, is now spreading to low- and middle-income countries. It has attained the status of a global pandemic, due to the phenomenon of Sanskritisation, a form of social shift where low-income groups attempt to move up the social scale by emulating the opulent practices of the affluent population.2,3 The two main drivers of the global rise in T2D are rapid urbanisation and obesogenic food environment.4 The top three countries with the greatest prevalence of diabetes in 2021 are China (140 million cases), India (74 million cases), and the USA (32 million cases).5 The absolute rise in people with diabetes in China and India is being driven by population growth and ageing in the past few decades.6 Mortality from communicable, maternal, neonatal, and nutritional diseases has halved; however, the number of death caused due to non-communicable diseases has significantly increased.7 In India, the burden of diabetes has increased significantly since 1990, and at a faster pace from the year 2000, as per the International Diabetes Federation (IDF). The mean healthcare expenditure on diabetes per person is 114 US dollars in India, and total deaths attributable directly to diabetes account for 0.6 million.8 Asian Indians represent an ethnic group with a significant propensity for developing T2D, and have high predilection to progress from pre-diabetes to diabetes at a younger age, and at lower levels of adiposity, as compared with White Europeans.9 The Asian Indian phenotype, identified by high levels of abdominal adiposity and increased insulin resistance, even at low levels of BMI, has been postulated as a risk factor for developing T2D.9
Dietary intervention is a cornerstone of diabetes management.10 Traditional dietary recommendations regarding quantity, order, and composition of meals to be consumed has been well established. However, the timing of food intake is also important for health. Chrono-nutrition is the circadian timing of meal intake. The circadian rhythms of the body are not precisely 24 hours and rely on Zeitgebers, such as exposure to light and food intake, to synchronise the circadian rhythm of the body.11 Sunlight is considered the most important Zeitgeber, as it influences the suprachiasmatic nucleus, or central clock, located in the anterior region of the hypothalamus, by providing the strongest signal for entrainment; it transmits the information to the peripheral clocks that are located in all the cells in the rest of the body.12 Feeding and fasting cycles, and dietary intake both have an impact on peripheral clocks. Nutrient timings can change the phase of the independently working liver clock.12 Ongoing research work suggests that the association between chrono-nutrition and health is likely analogous to those between nutrition and health. The link between circadian rhythmicity and metabolism has been studied in chrono-nutrition studies using animal models at various cellular, molecular, and behavioural levels.
Overall, laboratory findings suggest that chrono-nutrition plays a potential role in general wellbeing. There are six studied behavioural patterns likely to impact one’s chrono-nutrition status: night eating, time-restricted feeding, breakfast consumption, timing of the largest meal, timing of evening eating, and evening latency.12 In the area of chrono-nutrition, research on eating at night has helped to lay the groundwork for future dietary studies, by illuminating the potential health effects of eating at different times.
The invention of the electric light led to working or being awake at night hours, which has led to circadian disruption by modifying melatonin levels. Several studies have reported that melatonin levels are lower in night shift workers.13,14 Melatonin, or sleeping hormone, plays a central role in the regulation of circadian rhythms. Melatonin and cortisol secretions are dependent on photoperiods, which are modified by seasonal fluctuations. Melatonin secretion lasts for a longer time in winter, which lengthens the resting phase, while in summer, the activity phase lengthens due to long hours of sunlight.15
The term time-restricted feeding (TRF), which is one of the behavioural indicators of chrono-nutrition, is eating inside a predetermined window of time throughout a 24-hour day. Experimental studies of TRF are mostly carried out in animals. TRF aids in counteracting the detrimental effects of a high-fat diet in mice, on various metabolic health indicators. Despite identical caloric intake across experimental groups, mice with ad libitum access to food gained almost 30% more weight than mice with food access restricted to 8 hours.16 Similar to this, independent of the meal composition, an eating window of 9 hours during waking hours increased insulin sensitivity.17
In a pilot study, participants who were overweight and had a baseline eating window of 14 hours were instructed to limit their eating to 10–11 hours for 4 months. Participants reported better sleep quality, along with weight loss.18 Breakfast skippers had significantly higher HbA1c levels and higher BMI than breakfast eaters. After adjusting for age, sex, race, BMI, diabetes complications, insulin usage, depressive symptoms, perceived sleep debt, and caloric intake at dinner, chrono-nutrition behavioural indicator skipping of breakfast was significantly associated with higher HbA1C values (β=0.108, where β is regression coefficient; p=0.01).19 Breakfast skipping has been linked to an increased risk of obesity by 4.5 times.20 Breakfast skippers lack appetite signalling due to disrupted circadian rhythm. There was an independent association of skipping breakfast with poor glycaemic outcomes in individuals with T2D.21
Having lunch late (around 3 p.m.) has shown negative effects on gut microbiota diversity.22 Late diners (evening latency within 2 hours) have a deleterious impact on glucose tolerance, especially in G-carriers of the risk allele at MTNR1B rs10830963.22 It is possible that evening chronotypes, also known as night owls, are more prone than morning chronotypes, also known as morning larks, to consume a higher percentage of calories later in the day.22 Evening circadian typology is not genetic, but derives from unhealthy behaviours.22
It was observed that participants had more calories with less than 4 hours of sleep, and later bedtimes than control participants.23 Evening latency is the time duration between the last food intake and sleep onset, which may affect metabolic health. The preference for sleeping and waking hours or chronotype and health have both been associated with the timing of having the largest meal. People who ate the largest meal at breakfast or lunch had a lower BMI than people who had the largest meal in the evening.24 As hormones with circadian rhythms are synchronised by light, times suitable for feeding and fasting for the American and European populations cannot be applied to the South Asian population due to differences in environmental factors, such as latitude, seasons, and hours of day and night; and modifiable factors, like diet and lifestyles.
Therefore, it is important to understand the role of chrono-nutrition and chronotype on blood glucose outcomes among people with T2D, which may be one of the non-pharmacological strategies to better glycaemic outcomes, prevent complications, and improve quality of life. To the best of the authors’ knowledge, this is the first study to explore the association of chrono-nutrition status and chronotype with blood glucose outcomes in India among people with T2D. This proposed study aims to examine the associations among chronotype, chrono-nutrition, and glucose outcomes. The authors hypothesised that the morning chronotype, shorter eating window, not skipping breakfast, early dinner time, having the largest meal during daytime hours, and longer evening latency are associated with better glucose tolerance among people with diabetes.
MATERIALS AND METHODS
Sample Size
The required sample size of 227 patients with T2D was obtained using formula n=(3.84) pq/L2. The sample size for estimation of proportion was at 5% level of significance. P=15.9 (average percentage of males and females having diabetes as 15.1% and 16.6% respectively, as per National Family Health Survey [NFHS-5] district factsheet), and q=1–p. Absolute precision (L) was taken as 5%. Design effect was taken as 1.
Sampling Technique
The sample size was selected through random sampling.
Study Design
This study was a cross-sectional study, based in an urban city between June 2020–June 2021. Participants from three diabetes clinics were identified who met the study criteria.
Inclusion Criteria
Males and females, aged between 35–60 years, diagnosed with T2D in the past 0–5 years, without secondary complications, and willing to participate.
Exclusion Criteria
Participants with poor compliance for follow-up at the clinic.
Questionnaire
The following information was collected through a structured questionnaire using the interview technique.
Personal information
Personal information, including name, age, sex, education qualification, occupation, and family type was collected from the participants. Education qualification was classified according to the Kuppuswamy socioeconomic scale.
Anthropometric and body composition data
The weight was measured using standardised and well-calibrated Body Composition Monitor BodySCAN™ HBF-224 (Omron Healthcare, Kyoto, Japan). Height was measured using the standardised and well-calibrated portable stadiometer floor model with a 20–210 cm measurement range, and 1 mm graduation. Height was read to the nearest mm.
BMI is calculated as weight in kg divided by the square of the height in meters (kg/m2), and has been categorised into four groups according to the Asian-Pacific classification. BMI is calculated by dividing weight (in kg)/height (m²). Waist–hip ratio is calculated by dividing the waist measurement by hip measurement, with the formula waist circumference/hip circumference. According to the American Diabetes Association (ADA), the normal waist–hip ratio for Indian males is 0.88, and for females 0.81. Body fat and muscle percent were assessed using Body Composition Monitor HBF-375 (Omron Healthcare).
Diet information
This was collected using a 24-hour dietary recall for 3 days (2 weekdays plus one rest day). The 3-day 24-hour diet recall was converted into raw ingredients, and then quantified using the DietCal software (ProfoundTech Solutions, New Delhi, India).
Chronotype
This was assessed using the Horne and Östberg Morningness-Eveningness questionnaire (MEQ), which includes 19 questions to determine morningness-eveningness in human circadian rhythms. Scoring ranges from 16–86. Scores from 16–30 indicate ‘definitely evening’, 31–41 indicate ‘moderate evening’, 42–58 indicate ‘intermediate types’, 59–69 indicate ‘moderate morning’, and 70–86 indicate ‘definite morning’.
Biochemical parameters
Biochemical parameters, like HbA1c, fasting blood sugar, and post-prandial blood sugar collected from the patient’s case records of no older than 1 month, included the following chrononutrition profile. This was assessed by taking several behaviours in consideration:12 eating window, breakfast skipping, evening latency, evening eating, night eating, and largest meal are the behavioural indicators of chrono-nutrition.
Sleep
The Pittsburgh Sleep Quality index (PSQI) was used to assess the sleep quality among participants. The PSQI contains 19 self-rated questions. The scoring is divided into seven components, which have a range of 0–3 points: a score of 0 indicates no difficulty, while a score of 3 indicates severe difficulty. The score of the seven components are then added together, and one ‘global’ score is obtained that ranges from 0–21; here, 0 indicates no difficulty, and 21 indicates severe difficulties in all areas. A global score of 5 or more indicates poor sleep quality.
Triglyceride Glucose Index
The TyG index was calculated as the natural logarithm of the product of plasma glucose and triglycerides using the formula: natural logarithm (trigylcerides [mg/dL]×glucose [mg/dL]/2). It is a reliable and valid surrogate marker of insulin resistance.25
Data Analysis
The data was analysed in SPSS version 27 (IBM, Armonk, New York, USA). χ2 and Pearson’s correlation tests were applied.
Ethical Approval
The study was approved by the Institutional Review Board of the Department of Foods and Nutrition, Faculty of Family and Community Sciences, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat, India. The ethical approval number of the study is IECHR/FCSC/2020/46.
RESULTS
A total of 227 participants (35–60 years; 108 female) participated in the study. The study was carried out in two private diabetes clinics and one diabetes clinic in a tertiary private hospital in an urban area of Vadodara district, Gujarat, India. Around 53% and 31% of male and female participants have indicated graduation as their highest education qualification, respectively. None of them were illiterate. Around 88% of female participants were homemakers. Around 60% of male participants were retired. Around 73%, 22%, and 5% live in a nuclear family, extended family, and joint family, respectively. Most of the male participants (37.8%) and female participants (25.9%) are from the age group 55–60 years. The median duration of diabetes is 2 years. BMI, waist circumference, and waist to hip ratio are given in Table 1. Around 85% of the total male participants have more than 25% of fat. Around 65.0% of the total female participants had 30–35% of total body fat; 26.9% had more than 35% of total body fat, while only 4.6% had <20% of total body fat. Around 47% of total male participants and 31% of total female participants have visceral fat more than 15%. Most of the male (91%) and female (80%) participants have low total body skeletal muscle percentage of less than 24% of total body weight.
Chrono-nutrition status was assessed using six behavioural indicators that are likely to influence metabolic health: breakfast skipping, largest meal of the day, evening eating hours, evening latency, midnight eating, and eating window.
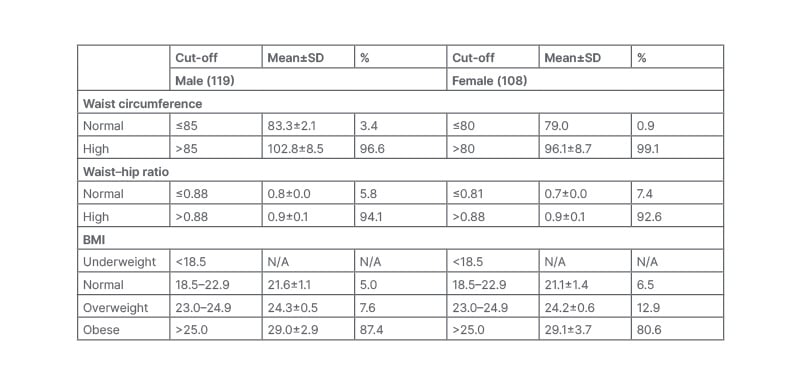
Table 1: Anthropometric parameters of the participants (n=227).
N/A: not applicable; SD: standard deviation.
There was a significant association between having a low glycaemic index (GI) dinner early with blood glucose levels (Table 2). There was a significant association between the greater frequencies of eating at midnight with poor glucose control (Table 2). The surrogate marker of insulin resistance TyG index correlated positively with the largest meal in the resting phase (r=0.209, where r is Pearson’s correlation; p=0.002). No one skipped breakfast more than once a week in the present study.
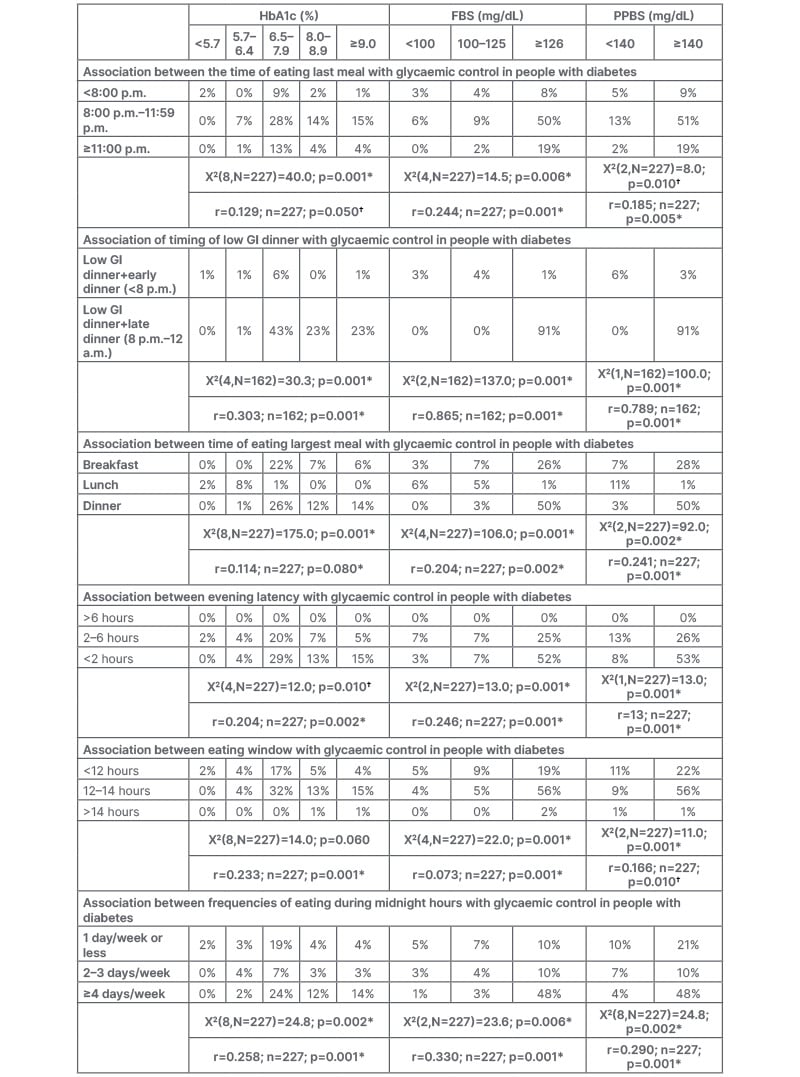
Table 2: Association of behavioural indicators of chrono-nutrition with glycaemic control.
*Highly significant
†Significant
A p-value less than or equal to the significance level (≤0.050) is statistically significant, while less than or equal to the significance level (≤0.005) is statistically highly significant.
FBS: fasting blood sugar; GI: glycaemic index; PPBS: post-prandial blood sugar; r: Pearson’s correlation.
A longer eating window negatively affected sleep (r=-0.123; p=0.05). There was a significant association between the longer evening latency with glucose homeostasis. Early dinner and longer evening latency aid in the glycaemic control of people with diabetes. Evening latency also affected sleep. Evening latency and sleep were significantly associated (X2[2,N=227]=8.7; p=0.01). The shorter the evening latency, the poorer the quality of sleep (r=0.185; p=0.005).There was a significant association between having largest meal during the day than night, with better glucose control.
Chronotype of the participants: 47.1% of males and 44.4% of females had the morning chronotype, while 52.9% of males and 55.6% of females had the evening chronotype. There was a significant association between morningness, with greater servings of vegetables, lesser sugar servings, and lesser servings of caffeinated beverages and sweets (Table 3). There was a significant association between morningness, with early dining, shorter eating windows, having the largest meal in the day, and lesser midnight cravings (Table 3). Participants with the evening chronotype ate almost twice the amount of carbohydrates and fat at dinner than at breakfast.
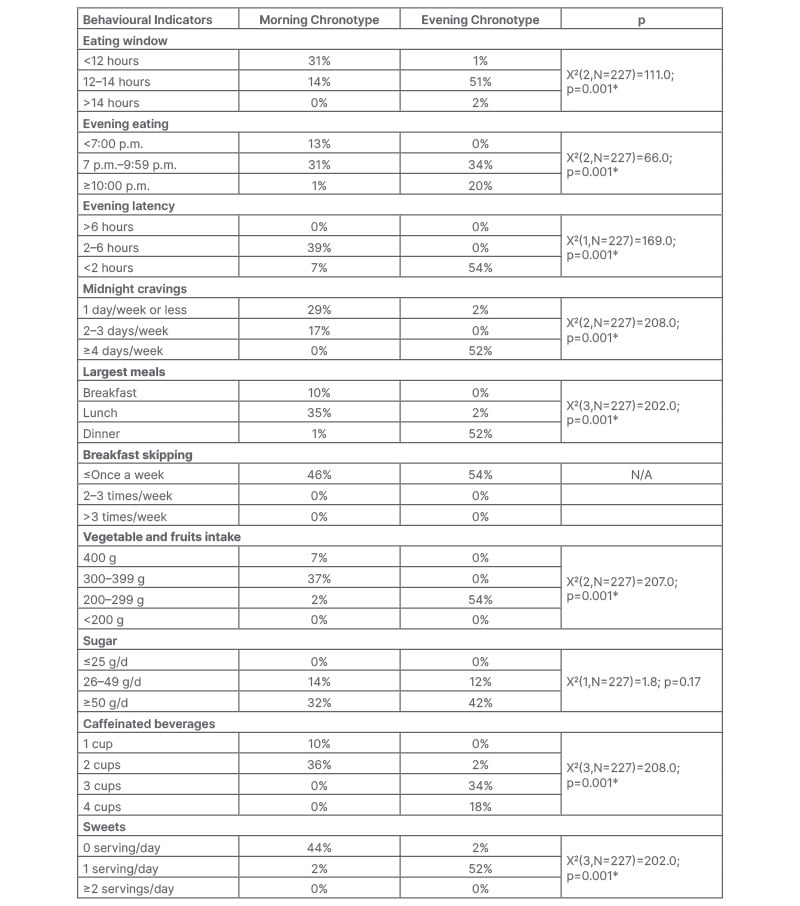
Table 3: Association between chronotype with chrono-nutrition components in people with diabetes, and association between chronotype with dietary data in people with diabetes.
*Highly significant: A p-value less than or equal to the significance level (≤0.005) is statistically highly significant.
N/A: not applicable.
DISCUSSION
Late-night eating has deleterious effects on insulin sensitivity.26 As reported in a few studies, glucose outcomes were better in the morning hours (8 a.m.) than in the evening (8 p.m.) for an identical composition of meal.27,28 The offset reduction in insulin levels, and the decreased β-cell sensitivity to glucose occur in the evening.28,29 Hence, the consumption of carbohydrate-rich meals in the evening would not be processed efficiently, and will further reduce insulin sensitivity. Eating dinner early is crucial for metabolic health, especially due to insulin and leptin. Food intake decreases and lipolysis increases when leptin peaks at approximately 7 p.m., and later declines. Leptin also inhibits fat accumulation.30 The resting phase begins when melatonin production starts, which reduces glucose tolerance.31 The nutrients from the food get effectively digested and metabolised when the food is consumed earlier in the day, rather than during the resting phase. Hence, dinner should be eaten early, irrespective of the GI of the meal.32,33 An increase in melatonin levels suppresses insulin sensitivity, resulting in impaired glucose tolerance in the midnight hours; hence, late-night eating should be avoided. There was a significant association between the shorter eating window with controlled fasting and post-prandial blood glucose. The production of fibroblast growth factor 21 and adiponectin, which encourage fatty acid oxidation, glycolysis, and limit fat formation, occurs between 8 a.m.–4 p.m.34 Food consumed during the insulin peak, i.e., between 2 p.m.–6 p.m., promotes fat accumulation.35 A peak release in the leptin hormone, which decreases appetite, occurs around midnight, while a trough occurs at noon,36 when the sun is highest in the sky in India, and the body is becoming active again. This explains why the largest meal should be had at noon, contrary to popular opinion; this needs to be explored. The zenith of the sun varies by latitude and season of the year. The level of cortisol in the blood, urine, and saliva increased by 50–75% between 30–60 minutes post-awakening, and is lowest from midday in both sexes on all days.37,38 Eating a large meal is not advisable when cortisol peaks. It is important to practice meditation, walk in nature, and eliminate waste, rather than indulging in a large breakfast when the cortisol peaks. A recent study reported that 32% of the total daily intake of calories was consumed in the late evening between 7 p.m.–11 p.m. by healthy individuals in Indian, with the median dinnertime being after 10 p.m.39 Dinner is the most important meal in family environments, especially in South Asia, and not restricted to weekends.
Commensality, the act of eating together provides opportunities for social integration, and facilitates communication. These findings are likely to have public health implications, as calorie-dense meals are often consumed during late evenings, which can desynchronise circadian rhythms. Intermittent fasting does not have any standardised protocol. The effects of people following late intermittent fasting patterns on metabolic health, in comparison to early eating patterns should be studied.
The assembly of the presented results suggests that the associations between chronotype, chrono-nutrition, and glucose outcomes is significant. Eating meals as per the circadian rhythm could be one of the non-pharmacological strategies that favours better blood glucose outcomes. As interventions of meal timings and their effects are limited to animals, studies should be designed with the human population, in order to clearly establish time-restricted feeding and fasting protocols, which can vary by latitude and season of the year.







