Abstract
Diabetes and its complications are main causes of morbidity and mortality among adults in the USA. An increase in the number of individuals with diabetes is primarily attributed to changes in dietary patterns including increased consumption of obesogenic foods and beverages. Many individuals who are overweight and obese show signs of insulin resistance and are at increased risk of Type 2 diabetes mellitus (T2DM) and cardiovascular disease. Lifestyle interventions (i.e., physical activity and nutrition) are the cornerstone of T2DM management and prevention. Prior research attests to the health benefits of consuming nuts, which have a substantial amount of mono- and polyunsaturated fatty acids, for individuals at risk for or with T2DM, and walnuts appear to be particularly promising. Walnuts are rich in nutrients, minerals, antioxidants, and vitamins that can contribute to improved cardio-metabolic risk factors in individuals at risk for or with T2DM. This review assesses the cardio-metabolic benefits of walnuts in T2DM. The authors’ review indicates that the reported effects of walnuts on glycaemic control have been inconclusive, with several studies showing association with improved glycaemic control while others show no effect. Despite their high energy density and potential to contribute to weight gain, the authors’ review suggests that walnuts can contribute to satiety without association with weight gain. This review also suggests that walnut consumption has been associated with improved low-density lipoprotein cholesterol levels and endothelial function but has not been associated with blood pressure improvement. Meta-analyses are warranted to quantitatively assess impact of walnut consumption on these cardio-metabolic risk factors in T2DM.
INTRODUCTION
In the USA, diabetes is a public health problem of epidemic proportions, affecting almost 35 million individuals.1 An estimated 88 million adults aged 18 years and older have prediabetes, yet only one of 10 persons with prediabetes is aware.1 Of persons with prediabetes, 15–30% are likely to develop Type 2 diabetes mellitus (T2DM) within 5 years.1 Diabetes complications include stroke, hypertension, cardiovascular disease (CVD), blindness, kidney disease, nervous system damage, limb amputations, and biochemical imbalances that can cause acute life-threatening events.1 Total medical costs, including lost work and wages, for persons diagnosed with diabetes are estimated at $327 billion.2 Medical costs for people with diabetes are more than double those for persons without diabetes.2 Rates of cardiovascular mortality are 2–4 times higher among adults with diabetes than among those without diabetes.1
Persons who are obese are over 7 times more likely to develop T2DM and cardio-metabolic complications than are persons who are a healthy weight.3,4 Excessive body weight is associated with insulin resistance.5 Insulin resistance is associated with hyperglycaemia, dyslipidaemia, and hypertension,5,6 which promote cellular-level alterations of vascular tissues, with resulting formation of atherosclerotic plaque that reduces endothelium-dependent vasodilation.7,8 Most deaths among patients with diabetes are caused by atherosclerotic CVD.9
Glycaemic control remains the basis of diabetes care. Co-management of cardio-metabolic risk factors and prevention of their consequences are also essential to improve long-term survival. A modest reduction of as little as 5–7% of body weight can significantly improve cardio-metabolic risk factors among those at risk for and with T2DM.10-13 Patients at risk for or with T2DM are typically advised to consume foods with a low glycaemic index (GI). Low-GI diets have been shown to improve serum lipid profiles, reduce C-reactive protein levels, and aid in weight management, reducing the development of T2DM and CVD.14,15 Walnuts have a low GI16 and are rich in nutrients, minerals, antioxidants, and vitamins that can improve cardio-metabolic risk factors. Walnuts are relatively high in mono- and polyunsaturated fatty acids (MUFAs and PUFAs), particularly α-linolenic acid and linoleic acid ( Table 1 ), which are known to have favourable effects on cardio-metabolic health.17-25 This review examines the cardio-metabolic benefits of walnuts (including improved glycaemic control, body weight, lipid profile, blood pressure, and endothelial function) in those at risk for or with T2DM.
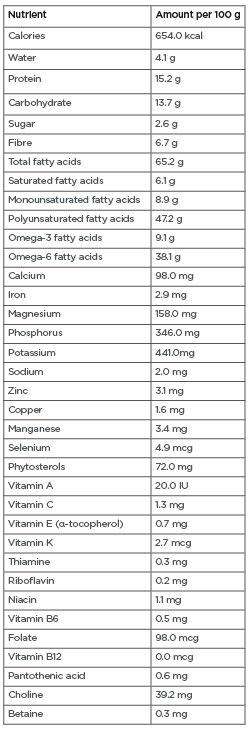
Table 1: Nutrient profile of walnuts.
METHOD
The authors reviewed all articles retrieved through Google Scholar and PubMed that were published from January 1999 to March 2021 using the following search terms: “walnut AND type 2 diabetes,” “walnut AND diabetes,” “walnut AND pre-diabetes,” “walnut AND prediabetes,” “walnut AND metabolic syndrome,” “walnut AND overweight,” and “walnut AND obesity.” Inclusion criteria included animal and human studies that were published in English; study population included those at risk for (i.e., prediabetes, metabolic syndrome, obesity, and overweight) or with T2DM; utilised walnuts as an intervention; and assessed at least one of the following parameters: glycaemic control, body weight, lipid profile, blood pressure, or endothelial function. Studies that did not fall within the timeframe of the review and not meeting the inclusion criteria were excluded.
GLYCAEMIC CONTROL
Walnuts are rich in MUFAs, PUFAs, and magnesium, which have been linked to improved glycaemic control, insulin sensitivity, and insulin-secreting capacity.24,25 Walnut inclusion in the diets of those at risk for or with T2DM has been associated with the displacement of high-carbohydrate foods.26.27 Displacing carbohydrates from diets while substituting MUFAs and PUFAs has been shown to improve blood glucose, insulin sensitivity, and insulin secretion.23,26In vitro, 10–100 µg/mL of walnut leaf extract has been linked to increased glucose uptake in cell-based assay and inhibition of protein tyrosine phosphatase 1B in a concentration-dependent manner compared to control cells.28 However, reported effects of walnut consumption on glycaemic control in T2DM have been considered inconclusive.
An epidemiologic study by Pan et al.29 that prospectively followed 137,956 females without diabetes, CVD, or cancer at baseline, aged 35–77 years, for 10 years reported that walnut intake of 1–3 servings/month (1 serving=28 g), 1 serving/week, or ≥2 servings/week was associated with lower T2DM risk compared with ˂1 serving per month. A cross-sectional analysis by Arab et al.30 of the population of the USA aged 18–80 years, using a sample of 34,121 individuals from the National Health and Nutrition Examination Survey (NHANES) database, reported a lower incidence of self-reported diabetes and better glycaemic control among those who consumed walnuts (i.e., 12 g or more based on a 24-hour dietary recall) compared with non-nut consumers.
A randomised controlled trial by Tapsell et al.31 with 50 patients with T2DM who were overweight, aged 33–70 years, reported reduced fasting insulin with daily inclusion of 30 g walnuts by those counselled to consume a low-fat, weight-maintenance diet for 3 months, compared with those counselled to consume a low-fat diet without walnuts. A randomised controlled study by Zibaeenezhad et al.32 with 100 patients with T2DM, aged 30–60 years, showed improved glycaemic control with inclusion of 15 g walnut oil daily for 3 months in their diets, compared with a control diet without walnuts. A randomised controlled study by Hosseini et al.33 with 61 patients with T2DM, aged 40–65 years, reported that 100 mg walnut leaf extract in capsules consumed twice daily before meals for 3 months improved glycaemic control compared with placebo capsules.
Conversely, a randomised controlled study by Rabiei et al.34 with 50 patients with T2DM, aged 30–80 years, reported that daily supplementation of 200 mg walnut leaf extract in capsules for 2 months did not improve glycaemic control or insulin sensitivity, although it reduced body weight and blood pressure compared with placebo capsules containing microcrystalline cellulose. Also, a randomised crossover-controlled study by Ma et al.35 with 24 patients with T2DM, aged 30–75 years, found that including 56 g walnuts daily for 2 months as part of an otherwise ad libitum diet with dietary counselling to regulate caloric intake did not show improved glycaemic control or insulin sensitivity. Likewise, randomised crossover-controlled studies by Katz et al.36 and Njike et al.37 with 46 and 112 participants, respectively, aged 25–75 years and at risk for T2DM, showed no association between glycaemic control and daily inclusion of 56 g walnuts in otherwise ad libitum diets, with or without advice to regulate caloric intake, for 2 months or 6 months, respectively, compared with ad libitum diets without walnuts. Brennan et al.38 studied 20 participants with metabolic syndrome, aged 40–75 years, and found no association between insulin resistance and consumption of 48 g walnuts in liquid meals for 4 days, compared with placebo.
In a randomised controlled study, walnut leaf powder administered twice daily at doses of either 25, 50, or 100 mg/kg for 28 days via gavage to 36 diabetic rats with ad libitum access to food and water improved glycaemic control compared to placebo (i.e., 10 mL/kg distilled water).23 In another randomised controlled study, 20 diabetic-induced female albino rats fed either 21.3, 42.6 or 85.2 g walnuts over 3, 7, and 10 days, respectively, showed improved glycaemic control compared with control diets without walnuts when assessed on those days.39,40
Overall, while several studies showed an association between improved glycaemic control and walnut consumption or administration, several other studies with more vigorous designs failed to show such association. Therefore, the effect of walnuts on glycaemic control in T2DM may be considered inconclusive (Table 2).26,27,29-43
BODY WEIGHT
The effect of walnut consumption on body weight has been linked to walnuts’ satiating effects. In a randomised controlled crossover study by Farr et al.,44 walnut intake for 5 days by participants at risk for T2DM was associated with reductions in perceived hunger and appetite and with increased activation of the insula (i.e., a region of the deep brain in the cerebral cortex) to highly desirable food signals. Brennan et al.38 reported that including walnuts in the diets of participants with metabolic syndrome for 4 days was associated with increased satiety without increasing hormones known to mediate satiety; this was likely due to an inadequate length of time to observe an increase in these hormones. In addition to their satiating effects, including walnuts in the context of a calorie-controlled diet has been associated with acutely enhanced oxidation of body fat in individuals who are overweight45 and with the displacement of high-calorie foods from diets.26,27
In a randomised controlled study with 50 patients with T2DM aged 30–80 years, 2 months of daily supplementation of 200 mg walnut leaf extract in capsules significantly reduced body weight compared with microcrystalline cellulose placebo capsules.34 In a secondary analysis of a randomised controlled study by Neale et al.27 with 337 participants who were overweight and obese, aged 37–51 years, including 30 g walnuts daily for 3 months in individualised diet plans with servings of each food group, combined with counselling to regulate caloric intake, was associated with significant weight loss compared with a control group receiving general advice for healthful eating and physical activity.
However, randomised crossover design studies by other investigators have found no association between weight loss and the daily inclusion of 56 g walnuts in otherwise ad libitum diets, or with counselling to regulate calorie intake diets, for 2 or 6 months in adults at risk for (n=46 and n=112) or with T2DM (n=24), aged 25–75 years, compared with a control otherwise ad libitum diet, with or without counselling to regulate calorie intake.35-37 In addition, in another randomised controlled study by Tapsell et al.41 of 58 patients with diabetes who were overweight, aged 35–75 years, a moderate-fat diet plan with advice to include 30 g walnuts daily for 6 months showed no association with weight loss compared with a control treatment plan utilising a standard clinical practice. In a randomised controlled trial with 100 patients with T2DM, aged 30–60 years, Zibaeenezhad et al.32 showed no body weight loss with daily inclusion of 15 g walnut oil for 3 months in their diets, compared to their exclusion.
Some animal studies have also examined the impact of walnuts on body weight. In a randomised controlled study by Mollica et al.,39 36 diabetic rats administered either 25, 50, or 100 mg/kg walnut leaf extract twice daily for 28 days via gavage and provided ad libitum access to food and water showed significant weight gain compared with placebo (i.e., 10 mL/kg distilled water). In another randomised controlled study by Onwuli et al.,39,40 diabetic-induced female albino rats fed either 21.3, 42.6, or 85.2 g of walnuts showed no association in body weight compared with control diets without walnuts at Days 3, 7, and 10 of assessment.
While two studies showed significant improvement in body weight, one small animal study showed weight gain with walnut inclusion in the diets, and an overwhelming majority of studies reported no association between weight loss and walnut inclusion in the diets. To be conservative with the inference of this review on the effects of walnuts on body weight, it is imperative to conclude that there is no association between walnut consumption and body weight in those with or at risk for T2DM ( Table 2 ).
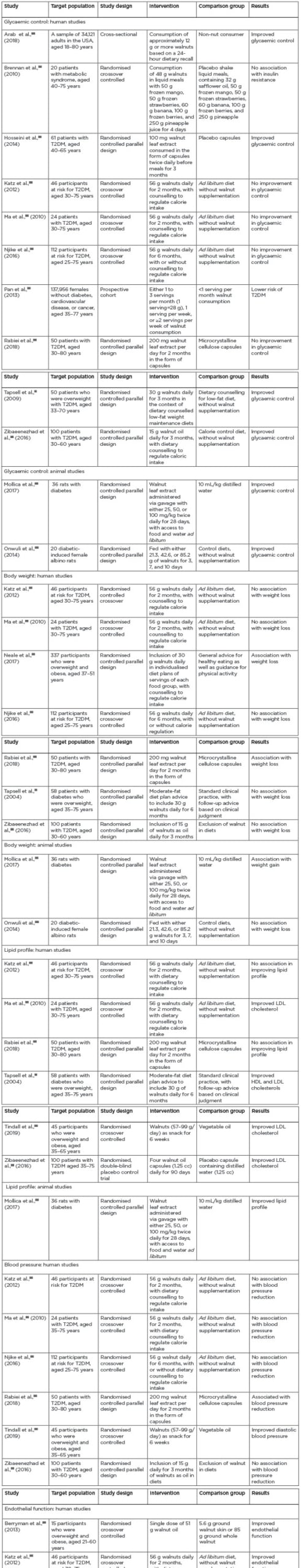
Table 2: Summaries of the articles included in the review. HDL: high-density lipoprotein; LDL: low-density lipoprotein; T2DM: Type 2 diabetes mellitus.
LIPID PROFILE
Daily inclusion of 48 g walnuts for 4 days by individuals with metabolic syndrome is associated with increased concentration of apolipoprotein A,46 which may ultimately lead to an improved lipid profile. This improvement may be due to the relatively high content of omega-3 fatty acids in walnuts. Dietary omega-3 fatty acids have been associated with reduced synthesis and secretion of very-low density lipoprotein and increased removal of triglycerides from very-low density lipoprotein and chylomicron through upregulation of lipoprotein lipase.47
A randomised control study by Tapsell et al.41 with 58 patients with T2DM, aged 35–75 years, reported increased high-density lipoprotein cholesterol and reduced low-density lipoprotein (LDL) cholesterol with the daily inclusion of 30 g walnuts for 6 months, together with counselling on a moderate-fat diet plan, compared with standard clinical advice without walnut supplementation. A randomised crossover-controlled trial by Tindall et al.42 also reported improved LDL cholesterol among 45 adults who were overweight and obese, aged 35–65 years, after replacing saturated fat with walnuts (57–99 g/day) as snacks compared with vegetable oils over a 6-week period. Other randomised controlled crossover studies (n=24 and n=112, respectively)35,37 also showed improved LDL cholesterol from baseline with daily inclusion of 56 g walnuts for 2 months and 6 months, respectively, in otherwise ad libitum diets, with or without counselling to regulate caloric intake of patients with or at risk of T2DM. Zibaeenezhad et al.32 reported improved LDL cholesterol with the intake of 4 walnut oil capsules (1.25 cc) daily for 90 days compared with placebo capsule containing distilled water (1.25 cc) during a randomised controlled trial among 100 patients with T2DM, aged 35–75 years. However, a randomised crossover-controlled study by Katz et al.36 with 46 participants at risk for T2DM, aged 30–75 years, did not report improved lipid profile with the daily inclusion of 56 g walnuts for 2 months in otherwise ad libitum diets, with counselling to regulate calorie intake, compared with ad libitum diets supplemented with walnuts.
Also, a randomised controlled study by Rabiei et al.34 with 50 patients with T2DM, aged 30–80 years, reported no improvement in lipid panel with supplementation of walnut leaf extract capsules compared with microcrystalline cellulose placebo capsules.
One randomised controlled study with diabetic rats by Mollica et al.23 assessed the effects of walnut leaf extract on lipid profile. The rats receiving either 25, 50, or 100 mg/kg walnut leaf extract twice daily via gavage for 28 days, with ad libitum access to food and water, showed significant improvement in their dyslipidaemia compared with those given 10 mL/kg distilled water as placebo without walnut leaf extract supplementation.
With only two studies showing no association between lipid profile and the inclusion of walnut in the diets, most of the studies showed a strong association. Therefore, it may be concluded that including walnuts in the diets of individuals with T2DM has favourable effects on lipid profile (Table 2).
BLOOD PRESSURE
The relatively high content of omega-3 fatty acids, polyphenols, L-arginine, and magnesium in walnuts may play an important role in improving blood pressure. Foods rich in omega-3 fatty acids lower blood pressure by upregulating angiotensin-converting enzyme-2.48 Foods rich in polyphenols act through the angiotensin-converting enzyme pathway by blocking this enzyme and therefore allowing blood vessels to relax, thus improving blood flow and reducing blood pressure.49,50 In addition, polyphenols stimulate the enzyme nitric oxide synthase to increase production of nitric oxide, which causes smooth muscles in blood vessels to relax, opening the arteries to improve blood flow. L-arginine is an amino acid used by the body to produce nitric oxide, which causes blood vessels to relax and dilate, thus lowering blood pressure.51-53 Magnesium stimulates production of prostaglandin E1, a potent vasodilator.54
In a randomised controlled study of 50 patients with T2DM, aged 30–80 years, Rabiei et al.34 demonstrated reduced office blood pressure with daily supplementation of 200 mg walnut leaf extract capsules compared with microcrystalline cellulose capsules. Likewise. Tindall et al.42 reported improved diastolic blood pressure in 45 adults who were overweight and obese, aged 35–65 years, when replacing saturated fat with walnuts (57–99 g/day) compared with vegetable oils over a 6-week period. However, other previous randomised controlled crossover studies (n=24, n=46, and n=112, respectively), with participants aged 30–75 years, have shown no association between reduced office blood pressure and the inclusion of 56 g walnuts in otherwise ad libitum diets, with or without counselling to regulate caloric intake in patients with or at risk for T2DM.32,35-37 A randomised control study by Zibaeenezhad et al.32 with 100 patients with T2DM, aged 30–60 years, showed no improvement in office blood pressure with the daily inclusion of 15 g walnut oil for 3 months in their diets, compared to diets without walnut oil supplementation.
All studies in this review (with the exception of just two studies) showed no association in improved blood pressure with the inclusion of walnuts in the diets. Therefore, it could be concluded that there is no association with the inclusion of walnuts in the diets of those with or at risk for T2DM (Table 2).
ENDOTHELIAL FUNCTION
Walnuts are rich in L-arginine amino acids, which are known to improve vascular function. In the body, L-arginine is converted into nitic oxide, a potent neurotransmitter that helps to relax blood vessels and improve blood flow.51-53 Also, walnuts are rich in polyphenols shown to have anti-oxidative effects in vitro and in vivo in mice with T2DM.55 Dietary polyphenol antioxidants bind to lipoproteins to inhibit oxidative stress processes that lead to the development of atherosclerosis.56 In addition, walnuts are rich in omega-3 α-linolenic fatty acids. Dietary omega-3 fatty acid intake has been inversely associated with the incidence of CVD. Omega-3 fatty acids are known to have an anti-inflammatory effect, which prevents the formation of pathological blood clots and also improves oxidative stress parameters.57,58
In the context of a randomised controlled crossover trial with 15 particpants who were overweight and obese, aged 21–60 years, Berryman et al.43 reported significantly improved endothelial function with the consumption of a single 51 g dose of walnut oil. In a randomised controlled crossover study by Ma et al.35 with 24 patients with diabetes, aged 35–75 years, including 56 g walnuts daily for 2 months in otherwise ad libitum diets with counselling to regulate calorie intake improved endothelial function compared with control ad libitum diets without walnut supplementation. Additionally, in other randomised controlled crossover studies, Katz et al.36 and Njike et al.37 (n=46 and n=112, respectively) also demonstrated improvement in endothelial function with the inclusion of 56 g walnuts daily for 2 months and 6 months, respectively, in otherwise ad libitum diets, with or without counselling to regulate calorie intake compared to ad libitum diets without walnuts in participants at risk for T2DM, aged 30–75 years.
This review suggests that all studies discussed here showed an association in improved endothelial function with the inclusion of walnuts in the diets. Therefore, it could be concluded that the inclusion of walnuts in the diets in patients with or at risk T2DM has favourable effects on endothelial function (Table 2).
DISCUSSION
Based on the body of evidence, the reported effects of walnut on glycaemic control in those at risk for or with T2DM remains inconclusive. Although epidemiologic studies, animal models, and some randomised controlled and mechanistic trials showed an association between walnut intake and glycaemic control in individuals at risk for or with T2DM, some randomised trials failed to demonstrate this association. The variation of results observed in these studies may be due to differences in doses of walnuts, types of walnuts, length of interventions, demographics of participants, timing of introducing walnuts into their diets, and/or different dietary patterns in the context in which they were introduced. Additionally, some studies may have been under-powered. Therefore, a much larger study or meta-analysis is warranted to clearly elucidate any association in glycaemic control with walnuts.
The inclusion of walnuts in the diets of individuals at risk for or with T2DM showed no effects on body weight in most of the studies. One study showed reduced body weight with the inclusion of walnuts in the diets. This difference in results observed may be due to the ways in which walnuts were introduced into the diets of the study’s participants. Some of the study’s participants included the walnuts as snacks in-between meals, while others included the walnuts with their meals. Also, none of these studies were designed with body weight as the primary outcome measure. The design of a study with body weight as a primary outcome measure in which walnuts are introduced as snacks in-between meals has the potential to improve satiety to foster reduced caloric intake and body weight.
Most studies showed an association between reduced LDL cholesterol and the inclusion of 30–56 g walnuts in the diets of persons with T2DM. In addition, one study also showed improved high-density lipoprotein cholesterol. One study showed no association in lipid profile with the inclusion of 56 g walnuts in the diets of persons at risk for T2DM. Different demographics of study participants and dietary patterns may explain the differences among the results. A study designed to examine differential effects of walnuts on lipid profile in participants with different demographic and dietary patterns is warranted.
Studies consistently showed no impact on blood pressure when introducing walnuts into the diets of persons at risk for or with T2DM. The lack of effects on blood pressure may be due to less reliable techniques used to assess blood pressure in these studies, insufficient doses, and the fact that blood pressure was not the primary endpoint of these studies. There is biological credibility that the biochemical composition of walnuts may reduce blood pressure. A study design in which blood pressure is the primary outcome measure and using more reliable techniques such as an ambulatory 24-hour blood pressure monitor to assess blood pressure is needed to elucidate the effects of walnuts on blood pressure.
Walnut consumption was associated with improvement in endothelial function in those at risk for or with T2DM in prior studies. A dose-response study is needed to determine the optimal dose and frequency of walnut consumption to maintain improved endothelial function throughout the course of the day, without adversely affecting other cardio-metabolic parameters.








