Meeting Summary
This article reviews an industry-sponsored satellite symposium that took place at the European Academy of Dermatology and Venereology (EADV) Congress 2024 held in Amsterdam, the Netherlands, on 27th September 2024.
The session, chaired by Khaled Ezzedine, Professor of Dermatology at Hôpital Henri-Mondor, France, addressed understanding the disease burden of vitiligo and the challenges of accessing optimal care. The session established vitiligo as an autoimmune disease requiring both early and long-term management, as well as utilising shared decision-making in treatment options.
Albert Wolkerstorfer, Professor of Dermatology at Amsterdam University Medical Centres, the Netherlands, discussed the underestimated burden of vitiligo disease, including psychological comorbidities, and the impact on quality of life (QoL) compared to other chronic diseases such as psoriasis. He also identified the challenges such as delayed diagnosis and lack of knowledge, and how this impacts access to optimal care.
Curtin Conrad, Professor of Dermatology and Head of the Polyclinic and Centre for Psoriasis Lausanne University Hospital, Switzerland, then considered the pathogenesis of non-segmental vitiligo, focusing on the role of the JAK-signal STAT pathway and how it drives the disease mechanisms and maintenance, emphasising the important need for early intervention and long-term considerations for the management of vitiligo.
Finally, Markus Böhm, Professor of Dermatology at the University Hospital Münster, Germany, identified the importance of utilising shared decision-making in vitiligo treatment strategies, especially for long-term commitment, and how ruxolitinib cream fits into this shared decision-making and overall treatment strategy, considering the efficacy and safety data.
INTRODUCTION
Vitiligo is a chronic autoimmune disease caused by a deregulation of the immune response that results in epidermal melanocyte destruction.1 With a variable and unpredictable disease course consisting of stable periods, spontaneous re-pigmentation, and new patches or flares, it should no longer be considered a cosmetic disease.
The characteristic depigmented lesions are often associated with psychological distress for patients, resulting in negative effects on mental health and QoL, with vitiligo being associated with social isolation, stigma, depression, and anxiety.2
How the Underestimated Vitiligo Burden Impacts Optimal Care
Albert Wolkerstorfer
Wolkerstorfer opened the session by considering the burden of vitiligo and what it is to live with vitiligo, including its impact on appearance, unpredictable flare-ups, itching, and association with autoimmune disorders. He said that patients are “desperate because of their vitiligo,” explaining that even those patients who look self-confident, self-assured, beautiful, and strong, “all had difficult times in life,” stating that “appearance does matter.”3 Wolkerstorfer supported this view by sharing data from a study that demonstrated that in those patients with facial abnormalities, such as scars, strangers scored photos of those with abnormalities as being judged more often as dishonest, unsuitable for employment, unintelligent, or unattractive compared to edited images without abnormalities.4
Living with the Burden of Vitiligo, Including Psychological Comorbidities
It is not only appearance that matters, but also unpredictable flare-ups and itching, the latter of which Wolkerstorfer stated occurred in one out of five patients. Those with flare-ups have a poorer QoL5 and are impacted by vitiligo disease progression, particularly in those that were stable and then rapidly depigment.6
A study from Belgium reported that 84.1% of patients were highly motivated to follow treatment if the stability of vitiligo could be attained.7 As such, this can be an important treatment goal, especially when the extent of vitiligo is not so large, i.e., when the affected body surface area (BSA) was less than 10%, and when the disease duration was less than 2 years, patients were more highly motivated to follow a treatment.7
The session also noted that healthcare professionals (HCP) do not fully understand the physical comorbidities of vitiligo, which include the association with thyroid disease and other autoimmune disorders, including alopecia areata, pernicious anaemia, and diabetes.7-9 Wolkerstorfer reported that, in his experience, it is not even known by dermatologists, for example, that sensorineural hearing loss is six times more likely in those with vitiligo compared to controls, as reported in a meta-analysis (odds ratio: 6.02; 95% CI: 3.41–10.62).10
Wolkerstorfer emphasised the impact of psychosocial comorbidities, which he referred to as “the dark side of white patches,” including depression, anxiety and anxiety-related disorders, stigmatisation, adjustment disorders, sleep disturbance, low self-esteem, and relationship difficulties.11
A large systematic review of 168 observational studies found 11 studies that reported depression was more likely to occur in patients with vitiligo compared to controls, 10 studies that showed an association to anxiety, and six for emotional and behavioural impairment.11 Considering depression, a systematic review and meta-analysis of observational studies reported that patients with vitiligo are approximately five times more likely to have depression compared to controls (pooled odds ratio: 5.05; 95% CI: 2.21–11.51).12
The session also highlighted the need for HCPs to look for signs of depression in their patients with vitiligo. A meta-analysis of 20 eligible cohorts (N=1,965 patients) identified a pooled prevalence of depression of 29% (95% CI: 20–39) in those with vitiligo compared with controls (across 17 unique populations [n=1,711]).13 Patients with vitiligo were 4.96 times (95% CI: 1.80–13.68) more likely to display depression, and sub-group analysis showed the prevalence was higher in female patients compared with males (standardised mean difference: 0.87; 95% CI: 0.52–1.22) and those of Asian ethnicity (pooled prevalence: 35% compared to 24% of Caucasians).13
Highlighting the global VALIANT study, an international cross-sectional online population-based survey of mental health conditions and psychosocial QoL burden among patients with vitiligo (n=3,541).14,15 The findings identified that diagnosed mental health conditions were common in 58.7% of patients with vitiligo, including anxiety (28.8%) and depression (24.5%).15 Moderate-to-severe symptoms of depression were common (55.0% globally), with the highest rates seen in India (89.4%).15,16
The study also indicated that the extent of vitiligo matters. The more extensive the affected BSA (more than 5%), the higher the rate of moderate-to-severe depressive symptoms (72.0%); the same was also seen for patients with darker skin types (68.3%; as per Fitzpatrick skin phototypes), and the presence of facial or hand lesions (64.4%).15
Parallels Between Vitiligo and Other Skin Disorders
Wolkerstorfer drew parallels to other chronic skin disorders like psoriasis and eczema, emphasising similarities such as chronic course,14 unpredictable flare-ups,5 physical comorbidities,8-10 psychosocial comorbidities,11 disease burden,11,15 and the need for long-term treatment.14
In comparison with other dermatologic conditions such as acne, alopecia areata, atopic dermatitis, psoriasis, urticaria, and eczema, vitiligo has similar, if not higher percentages of psychosocial comorbidities such as depression and anxiety.11
The session also noted differences in the evolution of skin disorder care. Until recently, the treatment response has been limited, and the primary endpoint of treatment has been at least vitiligo area scoring index 50 (VASI50) i.e., 50% re-pigmentation.17,18 Whereas in psoriasis the primary endpoint is typically psoriasis area and severity index 90 (PASI90) i.e., 90%.17,18 Also, treatment response is delayed, taking 6–24 months of treatment to achieve significant re-pigmentation.18 This,Wolkerstorfer said, “makes it quite frustrating, both for physicians and for patients.”
Another important difference, compared to nearly all other skin disorders, is that the skin has a loss of melanocytes,19 meaning that vitiligo can be particularly difficult to re-pigment in certain regions.
Access to Optimal Care and Hurdles in Vitiligo Care
Wolkerstorfer stated that “the patient journey starts long before the diagnosis,” and there are many issues along the patient journey which decrease access to optimal vitiligo care (Figure 1).20
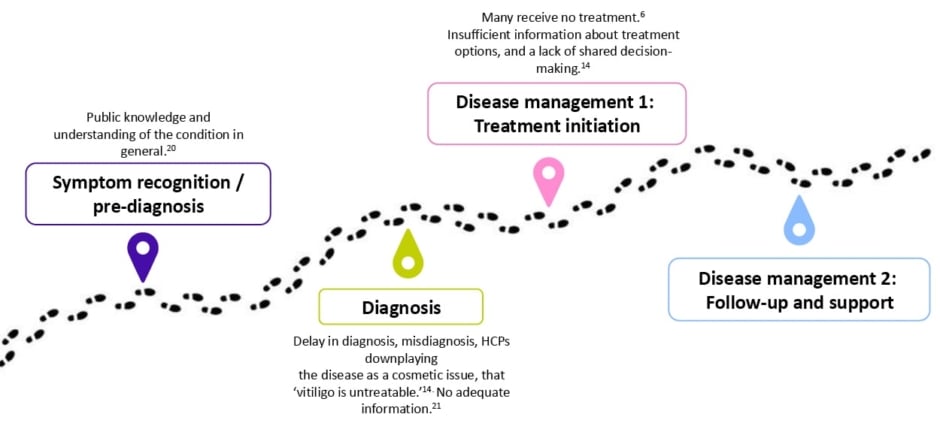
Figure 1: Issues along the patient journey.20
Access to specialist care and therefore treatment is poor for many patients across many countries. The VALIANT study, which surveyed skin disorders in Europe, the USA, and Japan (N=35,694 participants), estimated a 1.3% prevalence of vitiligo, with many patients undiagnosed (0.4%; including 0.3% vitiligo signs).6 Wolkerstorfer believes that the delay in diagnosis, which on average takes 2.4 years following the appearance of the first lesions,14 was due to a lack of HCP knowledge and awareness about the disease. Misdiagnosis is also very common, with 44.9% of patients reporting an earlier misdiagnosis.14
When there is a diagnosis of vitiligo, patients often hear that it cannot be treated. Of patients, 56.7% are told that vitiligo is untreatable, with higher rates among those with darker skin types (66%), those with extensive vitiligo affecting more than 5% BSA (65.2%), and those treated by a non-dermatologist (68.8%).14 This lack of knowledge of management options further adds to the burden of the disease, noted Wolkerstorfer.
A survey study conducted in the Netherlands (325 patients with vitiligo), considered the patients’ perspective regarding current treatments and the demand for novel treatments.21 The results indicated that half of the patients were dissatisfied with their current treatment, with 49% reporting that current treatments were not effective. Additionally, 94% of patients expressed the need for new and improved treatments.21
A statement from a patient who got frustrated with their healthcare experience said: “I went from dermatologist to dermatologist. Each told me there was no cure and no really effective treatment…seemed disinterested when I spoke of the dramatic change I saw in the mirror.”22
Changing the Trajectory of Vitiligo in Europe
The European white paper is a recent initiative aimed at identifying the gaps in the diagnosis, management, and care of vitiligo.23 A Delphi consensus, which gathered recommendations from patient organisations and experts, aims to improve health outcomes and access to optimal care.23 It included officially classifying vitiligo as a chronic autoimmune disease and calls on policymakers and European authorities to drive initiatives and facilitate best practices for sharing information, as a matter of urgency.23 A link for more information, which is available in the reference list, was provided.24
Wolkerstorfer concluded that the burden of vitiligo is significant, and though there are many parallels to other chronic skin disorders, some specific items make vitiligo very resistant and difficult to treat, and access to optimal care is sometimes very difficult, and there are some hurdles still to tackle.
As an Autoimmune Disease, Vitiligo Warrants Both Early and Long-Term Considerations
Curdin Conrad
Considering the treatment of chronic inflammatory diseases, Conrad highlighted the importance of finding treatment options that balance improving skin lesions while minimising side effects. He said this involved considering factors such as efficacy, benefits, risks, side effects, and inconveniences.25
The session emphasised the need to understand the pathogenesis of the deregulation of the immune system in order to understand the disease and then identify treatment opportunities. Non-segmental vitiligo is a chronic T helper (Th)-1 autoimmune disease.26 Conrad drew comparison to other immune diseases such as psoriasis, which is a Th-17 driven disease, and lupus which is driven by Type I-interferon; alopecia areata and vitiligo are driven by Th-1 via interferon-γ, which was demonstrated by the overexpression seen in gene expression profiles.
The Role of JAK-STAT Pathway Inhibition in Vitiligo
The pathogenesis of vitiligo is driven by auto-reactive CD8+ cytotoxic T cells that produce interferon-γ, which in turn signals the JAK pathway promote the production of IL-15 which is critical in the activation and maintenance of skin keratinocytes and relapses.26-28 Within these cells form tissue-resident memory T cells (TRM) in the lesions, which are recruited by the IL-15 signalling.26,28
Conrad expressed the challenges in eliminating TRM cells and the potential for preventing their entry into the skin through early intervention. Therefore, he suggested the role of IL-15 and interferon-γ as central in the pathogenesis of non-segmental vitiligo and identified the need to target these with treatment. However, he stated there is currently nothing to target Th-1 diseases.
As such, the session identified targeting the Th-1 cytokine receptors, IL-15 and interferon-γ, which are both important in the pathogenesis of alopecia areata and vitiligo, through the associated signalling JAK-STAT pathways.27,29
Interferon-γ receptor signalling in keratinocytes activates the JAK-STAT pathway via JAK1 and JAK2, resulting in transcription and production of CXCL9 and CXCL10, which induces CD-8 positive T cell recruitment which are capable of melanocyte destruction.30,31 By inhibiting JAK1, this would impact the response of TRM cells and interferon-γ.27,29
Conrad stated that by targeting these inflammatory processes we may diminish autoimmune attacks on melanocytes and lead to re-pigmentation over time, as damaged melanocytes are replaced by new melanocytes.2,31,32
Disease Course and the Memory of Vitiligo and Long-term Options
The challenge with current treatment options is that after successful therapy, such as phototherapy, many patients relapse within 12 months with recurrent depigmentation that typically occurs in the same lesions. This Conrad highlighted, indicated the “development of a disease memory within the skin.” He proposed that a longer disease duration may be a risk factor for (earlier) relapse. Therefore, with “early intervention, we have a higher chance to tackle that problem,” and have a higher chance of the patient going into remission and avoiding a relapse. Conrad said that this could potentially “reverse disease memory or prevent its establishment.”
The Role of Tissue Resident Memory T Cells in the Skin
The session also discussed the concept of disease memory, where TRM cells remain in the skin even when clinically healed or re-pigmented, or as seen in conditions such as psoriasis where you have clinically normal skin.33-36 Conrad provided an example of vitiligo, where initial T cell activation leads to melanocyte elimination and subsequent depigmentation. Although treatment may result in clinical normalcy and re-pigmentation, he stated that the TRM cells remain. Following treatment cessation, subsequent local reactivation of these TRM cells by a disease trigger can recruit further T cells, resulting in a clinical relapse.33-36 It was suggested that for disease modification and prevention of relapse, it would be necessary to eliminate these TRM cells. A mouse model demonstrated that targeting IL-15 signalling with an antibody blockade could potentially reverse chronic vitiligo.34 However, Conrad cautioned that eliminating all TRM cells may not only remove disease-specific cells but also those providing protection against pathogens.
In previously unaffected, non-lesional psoriasis skin, TRM cells accumulate over the course of the disease.37 Conrad identified that an alternative is to prevent TRM cells from populating the skin through early intervention. However, there is limited data on this.
Furthermore, the session noted the challenges with disease memory, not just T cell memory but also inflammatory memory through epigenetic changes.38-40 Conrad discussed the concept of epigenetic memory and its role in disease persistence and the need for long-term treatment options. For example, during infection, inflammatory cells such as monocytes undergo epigenetic modifications, such as through histone methylations or DNA methylations, which ‘train’ or ‘tolerise’ the cell.41 However, Conrad highlighted that you cannot reverse the damage from epigenetic changes.
As such, TRM cells are critical in mediating disease, therefore highlighting the importance of early interventions for the opportunity to bring about disease modification in certain patients. However, as identified by Wolkerstorfer, vitiligo is a chronic disease with patients having delayed diagnosis or late intervention, and therefore there is a need for long-term treatment. Given the safety concerns for JAK inhibitors and the need to evaluate the long-term risks and benefits in vitiligo,42 Conrad identified the need for alternative long-term treatment options.
Objectives for Long-term Vitiligo Treatment
The need to halt depigmentation, slow the disease progression, prevent relapses, and have time to induce re-pigmentation can take 6 months up to 2 years or more. Conrad concluded that the aim for early intervention, to enable disease modification by preventing or reversing the skin population of TRM and preventing or reversing epigenetic changes is still yet to be seen.
However, there is a need to tackle the burden of disease through early intervention and as early as possible so that we have treatment options available, and that there is a need for long-term management.
Considering the pathogenesis of vitiligo, the session identified potential treatment targets to block early depigmentation by targeting interferon-γ and to prevent relapses by blocking IL-15 to prevent TRM cell activation or maintenance, which can be achieved through JAK1 inhibition (Figure 2).2,30,43-50 Conrad summarised that it makes sense to inhibit Th-1 through JAK1 inhibition as an option to achieve vitiligo remission and long-term control.
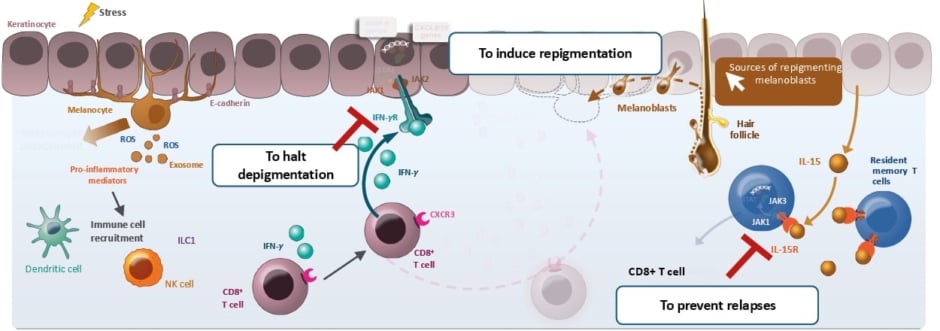
Figure 2: The vitiligo pathogenesis and objectives in vitiligo treatment.2,30,43-50
Figure adapted from Qi F et al.47 CC-BY-4.0
CD: cluster of differentiation; CXCL: C-X-C motif chemokine ligand; CXCR: C-X-C motif chemokine receptor; IFN: interferon; IL: interleukin; IL-R: interleukin receptor; ILC1: group 1 innate lymphoid cell; JAK: Janus kinase; MMP: matrix metalloproteinase; NK: natural killer cell; ROS: reactive oxygen species; STAT: signal transducer and activator of transcription; TRM: resident memory T cell.
How Ruxolitinib Cream Fits in Shared Decision-Making Strategies for Effective Vitiligo Management
Markus Böhm
In the final presentation, Böhm highlighted the importance of shared decision-making strategies in treating patients with nonsegmental vitiligo.
Shared decision-making is an important tool in long-term management of chronic conditions such as vitiligo.51 Put simply, Böhm said shared decision-making is the “provision of information to the patient, and also to listen to the patient.” This includes considering patient expectations, providing clinically relevant information, considering the nature of the disease, diagnosis and consequences, and treatment options, including what areas are difficult to treat, expectations of available therapies, and that some areas may not respond well.51
The session highlighted that the role of shared decision-making is important as it enables patients to better adhere to a given treatment, avoiding treatment failure and improving QoL.51 Böhm also noted the tools to support this, whether they are paper-based resources or mobile applications, the importance is to provide the information.
This was also highlighted by the recent treatment algorithm by the International Vitiligo Task Force (Figure 3), where under the established diagnosis there are defined treatment goals, expectations, and prognosis, shared decisions based on treatment pros and cons, disease impact, disease activity, and lesion location.52 These points, Böhm noted, are what should be explained to the patient, together with the patient, as well as discussion of the therapy options.52
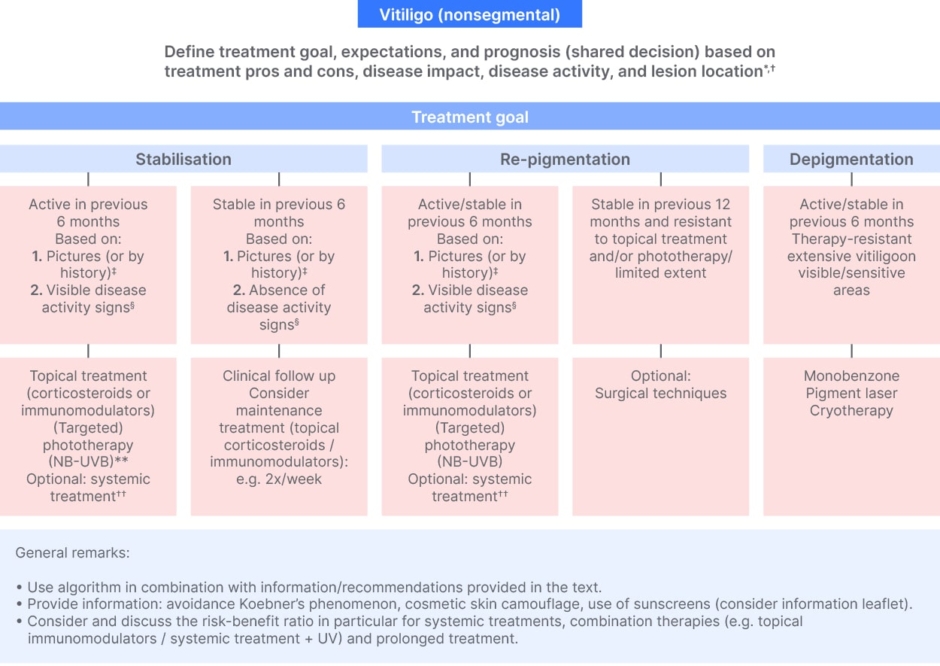
Figure 3: International statement of diagnosis and care of nonsegmental vitiligo.52
*Other aspects for shared decision: e.g., skin type, disease duration, presence of comorbidities, extent on visible/sensitive areas, geographical region.
†Explain relation between body location and expected results (‘best’ to ‘worse’: face > other body areas > hands/feet); explain the treatment expectations and limitations.
‡Active: new lesions or increase in existing lesions; stable: no new lesions or no increase in existing lesions.
§Clear presence of confetti-like depigmentations, hypochromic borders/areas or Koebner’s phenomenon (for assessment consider e.g., Br J Dermatol. 2020;183(5):883–890; www.vitiligo-calculator.com).
**NB-UVB and combination therapy preferred (e.g., phototherapy + topical corticosteroids).
††Oral steroid mini pulse (most investigated) and alternatives reported: methotrexate, cyclosporine, azathioprine, minocycline, and Janus kinase inhibitors (currently investigated).
Worldwide expert recommendations for the diagnosis and management of vitiligo: Position statement from the International Vitiligo Task Force Part 1: towards a new management algorithm, van Geel N, et al. © 2023 The Authors. Reproduced with permission of John Wiley & Sons Inc.
NB-UVB, narrow-band UVB.
van Geel N et al. J Eur Acad Dermatol Venereol. 2023.37:2173-84.
The session also emphasised the importance of HCPs knowing the clinical data of treatments to answer patient questions. Böhm stressed the significance of using a shared decision-making process to communicate to the patient.
Targeted Therapy for Vitiligo
To support HCPs’ understanding of treatment options, Böhm presented data from the first vitiligo-specific immunomodulatory therapy, a selective JAK1 and JAK2 inhibitor, ruxolitinib topical cream.
The two Phase III randomised controlled trials (TRuE-V1 and TRuE-V2) and long-term extension study (TRuE-V LTE) demonstrated the role of ruxolitinib, highlighting its effectiveness in achieving meaningful facial re-pigmentation (demonstrated by improved facial-VASI [F-VASI]) in long-standing vitiligo disease.53,54
These double-blind, randomised, vehicle-controlled studies recruited 674 patients (n=330 in TRuE-V1 and n=344 in TRuE-V2) aged 12 years and above with non-segmental vitiligo and a 10% or less BSA, from 101 centres in North America and Europe.53 The primary endpoint was an improvement in F-VASI75 (i.e., 75% re-pigmentation) after Week 24. Böhm noted this endpoint was selected, as previously identified by Wolkerstorfer, that facial depigmentation matters and is associated with the sites where patients are affected and stigmatised and is most important for the patient.
Those patients who were responders with F-VASI90 (i.e., 90% re-pigmentation) were invited to the extension and 2-year follow-up as part of the TRuE-V LTE.54,55 This cohort was randomised into two arms, one where ruxolitinib cream was applied throughout the trial, and the other was a withdrawal arm where patients stopped applying the treatment to evaluate relapse rate.54,55
Ruxolitinib Cream Patient Expectations as Part of Shared Decision-Making
The efficacy of ruxolitinib cream on the face after 6 months of continuous application achieved a meaningful endpoint of F-VASI75 by Week 52 in approximately half of the patients (50.3%), with one-third (30.3%) achieving F-VASI90.56
Böhm pointed out that this illustrated almost complete re-pigmentation of the face and non-facial areas such as the knee, highlighting the need to be consistent with treatment, manage patient expectations, and set individual patient goals.
Another point identified by Böhm is that when explaining clinical data to patients, to explain that it takes time. He said that unlike psoriasis or atopic dermatitis, with endpoints within 16 weeks or 24 weeks, it takes from 24 up to 52 weeks for vitiligo. Böhm indicated that if patients are not responding immediately, they should be persistent with the treatment.
The patient-reported outcomes included the vitiligo noticeability score, and factors such as skin colour matching and pattern of re-pigmentation to indicate treatment success. The response through 52 weeks indicated 36.3% of patients perceived their vitiligo as completely gone or almost completely gone.56
For those who achieved satisfactory re-pigmentation that continued into the extension trial (TRuE-V LTE), 69.1% of patients who continued application twice daily maintained an F-VASI75 response at Week 104.54 Among those patients who stopped treatment, an F-VASI75 response was maintained in 39.3%.54 Following treatment discontinuation, half of the patient’s relapse events occurred within 4 months.54
This emphasised, therefore, that continued application in the second year was associated with a reduced risk of losing an almost complete re-pigmentation of F-VASI90 response (hazard ratio: 0.32; 95% CI: 017–0.610).54 After stopping the treatment, the median time to maintain an F-VASI90 response was approximately 6.5 months.54 Böhm indicated, therefore, that if patients decide to continue treatment without stopping, efficacy is maintained.
In those patients who stopped the treatment application, perhaps those who wanted a treatment break after achieving satisfactory re-pigmentation, and then restarted (n=16), 75% were able to regain F-VASI75 within a median time of 12 weeks after restating, and approximately 70% regained a F-VASI90 after 15 weeks.54 In those patients who did not achieve satisfactory facial re-pigmentation in the second year (Weeks 52–104) of treatment, F-VASI90 still increased over time.55
Böhm highlighted that, based on shared decision-making, patients who may only achieve an F-VASI75 may decide to recontinue to achieve a re-pigmentation of 90%. Those who did not respond at Week 24 also showed improvement, with an increased F-VASI75 response, from 13.3% at Week 52 to 54.9% at Week 104.56
The proportion of patients who achieved a total VASI50 for all body regions (excluding the face), in all cohorts, increased from Weeks 52 to 104 with continued treatment application.57
Böhm stated that this indicated that for those patients who are not satisfied with their re-pigmentation should continue treatment. The data demonstrates that you can use continuous treatment to lead to better re-pigmentation, even for those with a poorer re-pigmentation rate.56
Safety Profile of Ruxolitinib Cream
When considering the treatment, patients may also want to understand the safety profile. Considering treatment-related treatment-emergent adverse events, the most common adverse event was application site acne, which was low, occurring in less than 6% of patients over 2 years.54,55,58 This, Böhm stated, indicated the treatment has a favourable efficacy and safety with a low incidence of adverse events.
Real-World Data Shared Decision-Making in Non-Segmental Vitiligo
Böhm also shared the first real-world data from Germany, showing the use of ruxolitinib cream in various patient cases, and discussed the importance of shared decision-making. In one case of non-segmental vitiligo, the patient considered the use of newer therapies to try and stop the disease progression and, with shared decision-making, considered the use on difficult-to-treat areas and the requirement of long-term treatment. In another case of long-standing vitiligo and slowly progressive disease, shared decision-making was used for considering alternative therapy options and the continuation of treatment on all sites to prevent relapse and promote further re-pigmentation in difficult-to-treat areas. A third patient case with progressive disease with particular concern for their legs used shared decision-making to continue treatment but stopped use on non-responding areas.
Böhm concluded that shared decision-making is an important tool for the successful therapeutic management of patients with vitiligo, particularly when considering newly approved therapies. Long-term treatment is usually needed in vitiligo, making shared decision-making an even more important tool considering the burden of using treatment against the need to get improved responses when treated for longer.
CONCLUSION
The burden of vitiligo and the challenges of accessing optimal care highlight the importance of both early and long-term management. Vitiligo is an autoimmune disease, and therefore, a better understanding of the pathogenesis focusing on the JAK-STAT pathway and the potential role of JAK-STAT inhibition for treatment options is important.
Dermatologists should be leading the frontier in the successful development of therapeutic management, with shared decision-making being one important cornerstone, in addition to effective treatment options, including ruxolitinib cream, considering efficacy and safety data. Overall, the symposium provided valuable information for HCPs to improve the management of vitiligo.






