Meeting Summary
The symposium “Pathways to silencing psoriasis: Remission or Cure?” took place during the 2019 European Society for Dermatological Research (ESDR) annual congress in Bordeaux, France. The presentations reviewed the role of the IL-23 pathway in psoriasis pathogenesis and other immune-mediated inflammatory diseases (IMID), underlined the importance of assessing and treating comorbidities in patients with psoriasis, and concluded with a glimpse into the future of psoriasis management, examining whether drug-free remission from disease is a viable goal for future treatment plans.
After defining and giving some examples of familial and poly-autoimmunity, Prof Jörg Prinz described the common pathways shared by several IMID. The involvement of the IL-23/Th(c)17 pathway in the pathogenesis of various IMID may represent opportunities for future therapeutic targets and treatment strategies.
The importance of holistic treatment in psoriasis management was illustrated by Prof Jo Lambert, who showed the audience how psoriasis can be linked to several different comorbidities, all of which should be addressed when making treatment decisions. Proper assessments and informed treatment choices could help patients with psoriasis achieve better clinical outcomes and help improve their long-term health expectations.
Reducing treatment burden for patients, and the possibility of achieving and maintaining drug-free remission, was discussed by Prof Carle Paul, who underlined the importance of examining several important predictive biomarkers of treatment response. Early, intensive treatment and disease modification could result in long-term remission of severe psoriasis and further decrease the treatment burden for patients.
The Role of IL-23 in the Pathogenesis of Psoriasis: A Common Pathway in Immune-Mediated Inflammatory Diseases
Professor Jörg Prinz
Psoriasis occurs more frequently alongside other IMID, indicating common pathogenetic pathways. Many of these IMID show familial accumulation
as a sign of a strong genetic predisposition. These phenomena have long been known as poly-autoimmunity and familial autoimmunity.1 Poly-autoimmunity refers to the persistence of multiple IMID in the same patient. Patients with one IMID are therefore susceptible to developing other IMID.1 Shared clinical and immunological characteristics of these IMID owing to overlapping aetiological factors reflect shared pathogenic pathways that may result in similar responses to the same therapeutic treatment.1
IMID are generally defined as a group of common and highly disabling chronic immune-mediated inflammatory conditions, characterised by a dysregulation of normal immune responses that lead to inflammation in the target organs and systemic effects. Over 80 IMID have been defined thus far, and their estimated current prevalence in Western society is approximately 5–7%.2,3 The most relevant IMID include: rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriasis, Crohn’s disease (CD), ulcerative colitis (UC), systemic lupus erythematosus (SLE), Type I diabetes mellitus, and multiple sclerosis (MS).4
IMID have complex genetic predispositions, and outside the major histocompatibility complex regions, each IMID shows >80 risk variants; however, the presence of genetic overlaps between IMID indicates possible similarities in pathogenetic pathways.4 For example, cross-trait analyses of multiple IMID have revealed that several of the diseases share several gene loci.5,6 Up to 85% of associated variants may be shared between different IMID, offering a glimpse into disease biology and directing common treatment approaches.5,6 Shared loci can have a concordant effect on disease manifestation, implying a shared risk of the disease, or a discordant effect, meaning that one locus indicates a disease risk factor despite having a protective function for a different disease.5,7 Importantly, concordant and discordant association of gene variants for psoriasis, AS, SLE, CD, UC, RA, MS, and coeliac disease are all related to the IL-23 and T helper (Th)1/Th17 pathways.5,7
Previous in vivo studies have indicated that the IL-23/17 pathways are involved in autoimmunity. Mice lacking IL-23, or have loss of IL-23 function, lack expression of IL-17-producing T cells and are resistant to developing several types of autoimmune diseases, including experimental autoimmune encephalitis and collagen-induced arthritis.8-10 Loss of function IL23R mutations in humans protect from psoriasis and psoriatic arthritis, CD, and AS. Thus, IL-23 and Th17 cells are implicated in the pathogenesis of various human autoimmune diseases. Accordingly, neutralising IL-23 specifically downregulates pathogenic Th/c17-cell activation and is highly effective in the treatment of psoriatic arthritis, AS, CD, UC, and SLE.4,8-10
In psoriasis, IL-23 maintains activation of the pathogenic T-cell response, stimulating keratinocyte proliferation.11,12 Blockade of the IL-23 pathway in patients with psoriasis is an effective and sustainable treatment, and efficacy appears to increase as interference with the IL-23/Th/c17 axis becomes more targeted.13-21 For example, recent results from the ECLIPSE clinical trial showed that treatment with guselkumab, an IL-23p19 inhibitor, resulted in superior long-term efficacy at 48 weeks based on Psoriasis Area Severity Index (PASI) 90 compared with secukinumab, an IL-17A inhibitor.22 Guselkumab treatment resulted in sustained PASI 90 scores over 48 weeks in 84% of patients, compared with 70% of patients receiving secukinumab.22 Similarly, results from the VOYAGE 2 trial showed that 88.6% of patients receiving guselkumab maintained PASI 90 responses at 48 weeks. Many patients showed a sustained effect of IL-23 blockade beyond discontinuation of the drug: 28 weeks after responders had been rerandomised to placebo, 62.0% still showed PASI 75, 36.8% PASI 90, and 19.0% were still free of disease.23
In conclusion, IMID are chronic inflammatory diseases with complex genetic predispositions. Shared genetic factors provide important insights into disease biology. Interfering with the IL-23/Th/c17 pathway provides control over various IMID associated with IL-23, resulting in novel treatment perspectives for diseases such as psoriasis, psoriatic arthritis, AS, UC, CD, and SLE. Thus, the shared genetic predispositions and disease pathways provide possible mechanistic links among IMID and represent valid and important therapeutic targets.
Optimising Psoriasis Care of Patients with Comorbidity
Professor Jo Lambert
Comorbidities associated with psoriasis can be classified into categories such as classic, emerging, lifestyle-related, or treatment-related, and can include such afflictions as cardiovascular disease, mood disorders, psoriatic arthritis, inflammatory bowel disease, malignancies, and kidney disease.24,25 Furthermore, cardiovascular disease is the leading cause of death in patients with psoriasis (Figure 1).25,26,27
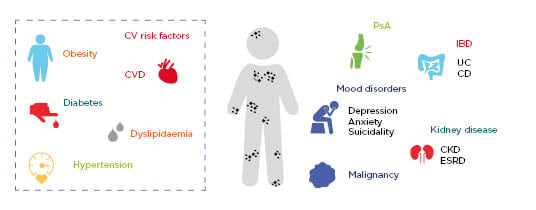
Figure 1: Psoriasis is associated with increased risk of serious comorbidities.
Comorbidities and risk factors associated with psoriasis.
CD: Crohn’s disease; CKD: chronic kidney disease; CV: cardiovascular; CVD: cardiovascular disease; ESRD: end-stage renal disease; IBD: inflammatory bowel disease; PsA: psoriatic arthritis; UC: ulcerative colitis.
Adapted from Takeshita et al.25 and World Health Organization.27
The importance of a multidisciplinary approach to psoriasis management emphasises the value of treating beyond the target of optimal skin care.28 Traditional screening methods include medical history, clinical exams, blood tests, and questionnaires, with defined cut-offs for specialist referrals, and use guidelines for age-appropriate cancer screening.25,29,30 Optimal treatment strategies rely on dermatologists’ awareness of psoriasis-related comorbidities, because the type of comorbidity can define later treatment decisions.31
Optimal treatment strategies should not only focus on the patient’s condition, but also address the patient’s needs and improve patient value.32 Patient value is particularly important, because there currently seems to be a disconnect between patient-perceived and medically perceived disease severity.33-35 A proposed novel approach to psoriasis management includes addressing the issue of undertreatment, taking an integrated approach to known health issues, screening for other psoriasis-related comorbidities, referring adequately, and paying more attention to the psychosocial and lifestyle-associated factors to ensure a full cycle of care for patients.33
Treatment decisions are ultimately the shared decision of the healthcare provider and the patient and should occur simultaneously while monitoring for comorbidities.36 The goals of treatment, therefore, should be to improve outcomes for the patient and improve patient and clinician experience, while keeping costs to a minimum.37 In the future, payment processes may move to a more ‘bundled payment’ plan for patients for psoriasis, shifting from fee-for-service practices to value-based reimbursement plans. The total payer cost would therefore focus on meaningful clinical outcomes for the patient and longitudinal, rather than acute, care programmes.32
The need to treat patients holistically and encourage them to adopt lifestyle changes to reduce the risk of other comorbidities is an important factor in psoriasis management; treatment choices can also have a substantial impact on psoriasis-related comorbidities and patient quality of life. In a recent study, patients treated with guselkumab showed significant improvements in general health-related quality of life, as well as significant decreases in anxiety and depression symptoms after 24 and 100 weeks of treatment.38,39 Another study, in a population of psoriasis patients at risk of coronary artery disease, showed how treatment with secukinumab may have a positive effect on cardiovascular health.40 Inhibition of the IL-12/23 pathway with ustekinumab has also been shown to transiently reduce aortic vascular inflammation in patients with psoriasis.41 Furthermore, comorbidities can also have an impact on the effectiveness of treatment. Results from a recent study showed that obesity can negatively impact treatment responses to anti-TNF agents.42
In conclusion, the importance of comorbidities in psoriasis management should not be underestimated. Taking these factors into consideration could result in better treatment outcomes for patients, and help patients improve their long-term health expectations.
Future of Psoriasis: Can Lasting Improvement be Achieved in Psoriasis Care?
Professor Carle Paul
Though many steps have been made in the past to improve psoriasis treatment, psoriasis management should focus on the need to decrease the treatment burden for patients, and to help patients achieve both long-term clearance and remission of psoriasis. Results from recent studies have shown that newer biologic therapies have resulted in increased efficacy in patients with psoriasis.43 Data from the BADBIR Registry and DERMBIO studies show that IL-12/23 inhibition results in higher levels of drug persistence and drug survival, compared with anti-TNF alpha or anti-IL-17 therapies.44,45 Furthermore, data from the PSOLAR study shows that drug persistence with ustekinumab was higher when given either as first, second, or third-line treatment, compared with infliximab, adalimumab, or etanercept.46
However, the data from real-life clinical practice does not always mirror clinical trial results, reflecting the need for a holistic approach to psoriasis and comorbidity management, and recognition of the fact that adherence to long-term treatment represents a challenge for some patients. Therefore, the PSTELLAR study aimed to examine the effect of dosing interval extension beyond 12 weeks on the efficacy of ustekinumab in subjects with moderate-to-severe plaque-type psoriasis.47 An assessment of Physician’s Global Assessment (PGA) scores showed that a subset of patients was able to maintain clearance while receiving a dose every 24 weeks,48 indicating that a decrease of treatment burden for patients can be achieved while still effectively maintaining a clinical effect.
Furthermore, studies with guselkumab show that >85% of patients maintained PASI 90 scores, and almost 60% of patients maintained PASI 100 scores 28 weeks after treatment cessation.23 Results from the VOYAGE-2 study led to the identification of important predictors for drug-free remission, including shorter disease duration and lower level of serum IL-17F (Figure 2) but also PASI 100 achievement at Week 28; Investigator’s Global Assessment (IGA) 0 achievement at Week 28; and higher blood guselkumab concentration at Week 28. The greatest amount of predictive power for drug-free remission of psoriasis was obtained using a model combining disease duration, IGA 0 response, and guselkumab concentration at Week 28.49
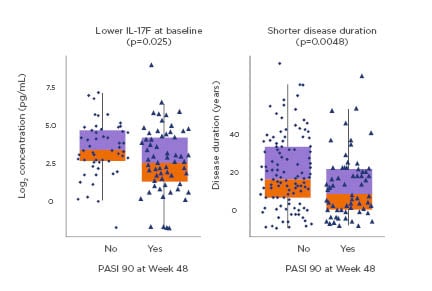
Figure 2: Predictors of drug-free remission after withdrawal from treatment with guselkumab.49
PASI: Psoriasis Area Severity Index.
Adapted from Liu et al.49
The GUIDE study will examine further maintenance treatment strategies with guselkumab by comparing several patient populations, including patients with short versus long disease duration and those with biomarkers identified as predictors of drug-free remission in the VOYAGE 2.50
For several reasons, the goal of obtaining remission in psoriasis represents a substantial hurdle for dermatologists. Epidermal Th22 and Tc17 cells can form localised ‘disease memory’ in clinically healed psoriasis plaques, and can produce cytokines associated with lesion formation, leading to relapses.51 Triggering of IL-17A resident memory T cells can also result in tissue-specific disease responses, and relapse, in previously resolved plaques.52 However, early, intensive treatment and modification of the long-term disease course may result in remission of severe psoriasis.53 Disease modification may offer the possibility to decrease, or discontinue, biologic treatment if the patient shows complete psoriasis clearance and no psoriatic arthritis development, has a normal body weight, and has recent onset of psoriasis.53 In Prof Paul’s opinion, current evidence appears to indicate that IL-23 inhibitors may lend themselves more easily to treatment reduction, or cessation of treatment, compared with TNF agonists and IL-17 antagonists.
In conclusion, numerous therapeutic options have helped to drastically improve psoriatic care, but the possibility to lower the burden of treatment for the patients should be evaluated in prospective clinical trials. The possibility of achieving long-term disease remission is being assessed in targeted populations and may represent opportunities for disease control beyond symptom management alone.







