Presenters: Matthias Augustin,¹ Diamant Thaçi,² Andreas Pinter,³ Kristian Reich,⁴ Jo Lambert,5,6 Peter Van de Kerkhof⁷
1. German Center for Health Services Research in Dermatology (CVderm), Institute for Health Services Research in Dermatology and Nursing (IVDP), University Medical Center Hamburg-Eppendorf, Hamburg, Germany
2. Institute and Comprehensive Center for Inflammation Medicine, University of Lubeck, Lubeck, Germany
3. Department of Dermatology, Venereology and Allergology, Clinical Research Division, University Hospital Frankfurt am Main, Frankfurt am Main, Germany
4. Translational Research in Inflammatory Skin Diseases, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
5. Department of Dermatology, Ghent University Hospital, Ghent, Belgium
6. Center for Inflammatory Skin Diseases, Department of Dermatology, Venereology, and Allergology, University Medical Center Schleswig-Holstein, Kiel, Germany
7. Department of Dermatology, Radboud University Medical Center, Nijmegen, the Netherlands
Disclosure: Prof Augustin has disclosed associations with AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen, LEO Pharma, Medac, Merck & Co., MSD, Novartis, Pfizer, UCB, and Xenoport. Prof Thaçi has disclosed associations with AbbVie, Almirall, Bioskin, Boehringer Ingelheim, Celgene, Dignity, Galapagos, GlaxoSmithKline, Janssen, LEO Pharma, Eli Lilly, Medac, MSD, MorphoSys, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi, Sun Pharma, and UCB. Dr Pinter has disclosed associations with AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Eli Lilly, Galderma, Hexal, Janssen, LEO Pharma, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz, Schering-Plough, and UCB. Prof Reich has disclosed associations with AbbVie, Affibody, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Eli Lilly, Forward Pharma, Fresenius, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Medac, MSD, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Takeda, UCB, Valeant, and Xenoport. Prof Lambert has disclosed associations with AbbVie, Celgene, Janssen, LEO Pharma, Eli Lilly, Novartis, and Pfizer. Prof Van de Kerkhof has disclosed associations with Abbott, Almirall, Amgen, Bristol Myers Squibb, Celgene, Centocor, Dermavant, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Philips, and Sandoz.
Acknowledgements: Writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Support: The publication of this article was funded by Almirall. The views and opinions are those of the authors and not necessarily those of Almirall.
Citation: EMJ Dermatol. 2021;9[Suppl 1]:2-11.
![]()
Corrigendum: Long-term Efficacy and Safety of Tildrakizumab: 5-year Results from the reSURFACE 1 and 2 Phase III Trials in Patients with Moderate-to-severe Plaque Psoriasis
Presenters: Matthias Augustin, Diamant Thaçi, Andreas Pinter, Kristian Reich, Jo Lambert, Peter Van de Kerkhof
Original citation: EMJ Dermatol. 2021;9[Suppl 1]:2-11.
Date correction published: 16.02.2021
The article by Augustin et al. in Suppl 1 of EMJ Dermatology 9.1 (pages 2-11) was originally published on 10.2.2021. Since then a corrigendum has been made. In the Conclusion, the phrase “the efficacy of tildrakizumab in achieving and maintaining control of psoriasis over 244 weeks” originally read “over 144 weeks” incorrectly. This has now been updated.
The EMJ apologises for the error and any inconvenience caused.
![]()
Meeting Summary
Psoriasis, a chronic systemic inflammatory disease with skin manifestations, can be an impediment to daily life. Accordingly, treatment for psoriasis needs to be efficacious and safe over an extended time period. IL-23 is a key upstream regulatory cytokine in the pathogenesis of psoriasis, driving the inflammatory cascade and hence playing a role in the manifestation of the erythrosquamous plaques indicative of this condition. IL-23 signalling can be blocked by tildrakizumab, a high-affinity anti-IL-23p19 recombinant monoclonal antibody. At the 2020 European Academy of Dermatology and Venerology (EADV) Virtual Congress, data for 5-year efficacy and safety of tildrakizumab in adults with moderate-to-severe plaque psoriasis were presented across six posters. Analysis of pooled efficacy data from the reSURFACE 1 and reSURFACE 2 trials showed that patients who had achieved clinically meaningful improvements at Week 28 continued to maintain responses over 5 years while receiving tildrakizumab 100 mg or 200 mg maintenance dosing. Rates of severe infections, confirmed major adverse cardiovascular events (MACE), and malignancies were all found to have low exposure-adjusted incidence rates, which were similar across tildrakizumab doses. Additionally, safety data were comparable in those aged ≥65 years, an age group with higher rates of comorbidities. Overall, these analyses show that tildrakizumab has a very favourable long-term efficacy and safety profile for the treatment of people with moderate-to-severe psoriasis.
Overview
While psoriasis is a systemic inflammatory disease, it predominantly manifests in the skin as thick, scaly, erythematous plaques.1 Both patient and physician surveys alike reveal that, over the long term, factors deemed important when considering a psoriasis therapy include the appearance and amount of clear skin, overall symptom relief, maintenance of response over time, and safety.2,3
A number of systemic therapies for psoriasis have been developed that target the biological pathways involved in the development of this condition.1 Three such biologics, the recombinant, high-affinity monoclonal antibodies tildrakizumab, guselkumab, and risankizumab, target IL-23, a cytokine produced by dermal dendritic cells that is involved in Th17 cell differentiation and survival.1,4,5 These differentiated Th17 cells produce an array of cytokines, including IL-17A, IL-17F, and IL-22, that drive inflammation, stimulate keratinocyte proliferation, and lead, among other pathways, to the erythrosquamous plaques indicative of psoriasis.4,5 Stimulated keratinocytes can themselves produce IL-23, creating a feedback loop between these and Th17 cells.4,6 The molecular target of tildrakizumab is IL-23p19, one of two subunits that form IL-23.7-9 By acting on this subunit, the differentiation, and thus downstream activities, of Th17 cells can be blocked, leading to long-term symptom control.4-8
Clinical trials for tildrakizumab include the reSURFACE 1 and 2 studies in adults with moderate-to-severe plaque psoriasis that affected ≥10% of their body surface area and who had a Physician’s Global Assessment (PGA) score ≥3 and a Psoriasis Area and Severity Index (PASI) score ≥12.9 At the EADV Virtual Congress, six posters were presented with the objectives of assessing 5-year efficacy and safety data of tildrakizumab.
reSURFACE 1 and reSURFACE 2 Study Design
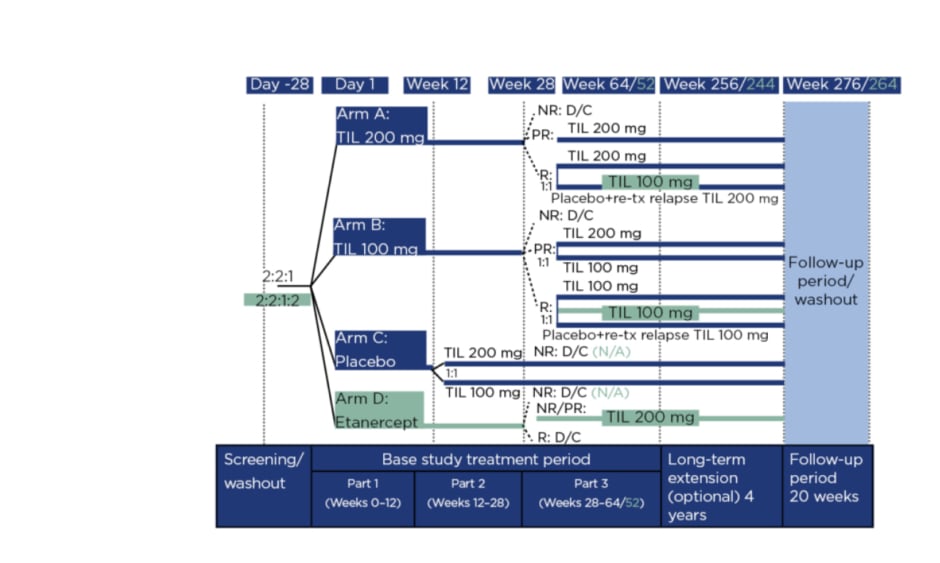
Figure 1 details the reSURFACE 1 and 2 trials. In Part 1, participants were randomised to tildrakizumab 200 mg (‘TIL 200’ group; n=616), tildrakizumab 100 mg (‘TIL 100’ group; n=622), or a placebo (n=311), all administered at 0, 4, and 12 weeks, with a 50 mg etanercept arm (n=313) added in reSURFACE 2, administered twice per week for 12 weeks.8 In Part 2, placebo arm participants were rerandomised to TIL 200 or TIL 100. Here, tildrakizumab was administered at Week 24 and etanercept once per week.
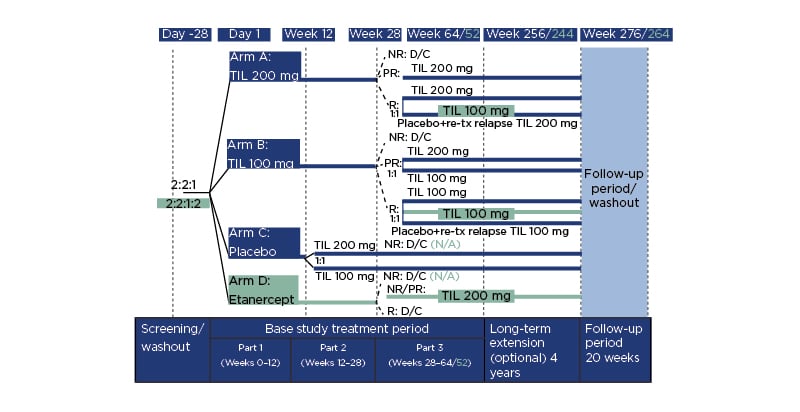
Figure 1: Study design.
Differences in design for reSURFACE 2 versus reSURFACE 1 are shown in turquoise. NR reflects <50% improvement in Psoriasis Area and Severity Index (PASI 50). PR reflects PASI 50–74%. R reflects PASI ≥75%. D/C: discontinued; N/A: not applicable; NR: nonresponders; PR: partial responders; R: responders; TIL: tildrakizumab; tx: treatment.
In Part 3, tildrakizumab responders (≥75% improvement in PASI score: PASI 75) in reSURFACE 1 were rerandomised of tildrakizumab to continue with the previous (or original) dose or to a placebo, with retreatment of 200 mg tildrakizumab if a relapse occurred. In reSURFACE 2, TIL 100 responders continued on this dose, with TIL 200 responders rerandomised to TIL 200 or TIL 100. Partial responders (PASI improvement 50–74%) who recieved TIL 200 continued on that dose, while TIL 100 partial responders were rerandomised to TIL 200 or TIL 100. Partial or nonresponders (PASI improvement <50%) to etanercept underwent a 4-week washout period then received TIL 200 at Weeks 32 and 36, then every 12 weeks. Tildrakizumab nonresponders and etanercept responders were discontinued.9 From Part 2, tildrakizumab was administered every 12 weeks and etancercept once a week.
Following the initial studies, those who completed Part 3 with a PASI ≥50 were invited to enter a 4-year extension 192 weeks. Participants remained on the same dose of tildrakizumab and received one injection at the start of the extension period, then one injection every 12 weeks.
Long-term Maintenance of Response with Tildrakizumab
Interim results of tildrakizumab efficacy in these trials has been reported previously over a number of time periods.9 Of those who responded at Week 28, approximately 90% in the TIL 100 or TIL 200 groups maintained PASI 75 status through Week 148. Those who achieved PASI 90 (approximately 70% at all time points) or PASI 100 (approximately 30% at all time points) status at Week 28 also maintained these levels of response at Week 148.8
For the efficacy posters presented at the 2020 EADV Virtual Congress, the objective was to evaluate maintenance of response through 244 weeks of treatment in patients who had achieved such by Week 28 and who remained on the same tildrakizumab dose throughout the trials. Two post hoc analyses were carried out: the first examined pooled reSURFACE 1 and 2 data and included all participating centres in Australia, Austria, Belgium, Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, Japan, North America, the Netherlands, and Poland;10 the second, which examined reSURFACE 2 data only, included only those in the European countries.11 For both analyses, missing data were imputed using multiple imputation.
Maintenance of Efficacy with Tildrakizumab Over 5 Years Is Shown in People with Psoriasis Who Achieved PASI <3 Response at Week 28
Professor Matthias Augustin
The post hoc analysis of all countries taking part in the trials included 194 participants receiving 200 mg tildrakizumab and 280 participants receiving 100 mg tildrakizumab who had achieved an absolute PASI score <3 at Week 28. The objective was to evaluate maintenance of this score over 5 years of continuous treatment. Baseline characteristics were similar for both TIL 200 and 100 groups (mean age: 45.9 and 44.8 years, respectively; standard deviation [SD]: 13.8 and 13.8, male: 67.0% and 68.2%, and mean PASI score at baseline: 19.4 and 19.2; SD: 7.2 and 6.8).10
As can be seen in Figure 2, by Week 52, PASI <3 was maintained in >90.0% of participants in the TIL 200 or TIL 100 groups.10 By Week 148, PASI <3 was maintained in approximately 85.0% of participants for both groups and at Week 244, representing 5 years of treatment, 88.3% in the TIL 200 group and 84.5% in the TIL 100 group maintained an absolute PASI score <3.
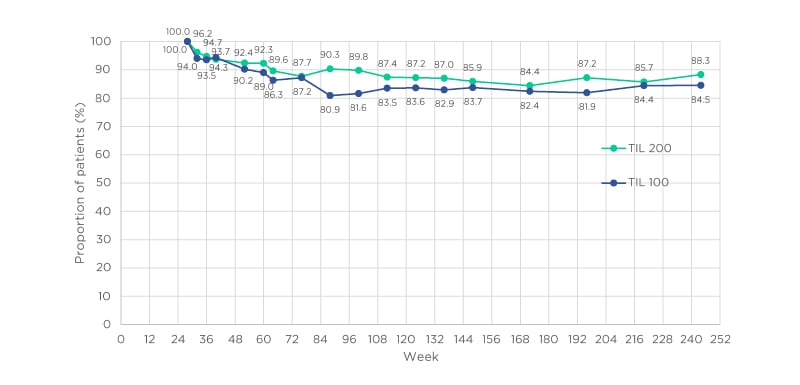
Figure 2: Evolution of Psoriasis Area and Severity Index (PASI) <3 response at Weeks 28–244 for tildrakizumab 100 mg and 200 mg groups.
Missing data were imputed using multiple imputation. TIL 100: tildrakizumab 100 mg; TIL 200: tildrakizumab 200 mg. Adapted from Augustin et al.10
Efficacy of Tildrakizumab Over 5 Years in European Centres
Professor Diamant Thaçi
For the 5-year efficacy post hoc analysis of participants in reSURFACE 2 from European countries only, the cohort comprised those who achieved PASI 75 by Week 28, as per the study design. In this analysis, 65 people received 200 mg tildrakizumab and 141 received 100 mg tildrakizumab. Baseline mean ages were similar to the entire cohort (mean ages: 44.4 and 43.6 years, respectively; SD: 13.1 and 12.9); however, a higher percentage were males (68.2% and 67.0%, respectively, in the all-centre analysis; 73.9% and 76.6% in the European cohort) and PASI score for the European cohort (20.8 and 20.5; SD: 7.3 and 7.2) was higher than for the entire cohort (19.2 and 19.4; SD: 6.8 and 7.2).11
As shown in Table 1, for both TIL 200 and TIL 100 groups, PASI 75 responses were maintained in >90% of participants at all time points, including at 244 weeks.11 PASI 90 was maintained or achieved by >60% of those in the TIL 200 group and 69% of those in the TIL 100 group at 244 weeks. The PASI <3 scores show that this level of response was maintained or achieved by most participants throughout the study, with approximately 80% in both groups maintaining PASI <3 at 244 weeks. Higher disease resolution, shown by PASI <1, was maintained or achieved in approximately one-half of the participants by 244 weeks, with little difference between TIL 200 and TIL 100 groups.
Table 1: Evolution of Psoriasis Area and Severity Index (PASI) score responders in European patients.
Percentages of patients achieving response. Missing data were imputed using multiple imputation. PASI: Psoriasis Area and Severity Index; PASI 75: ≥75% improvement in Psoriasis Area and Severity Index score; PASI 90: ≥90% improvement in Psoriasis Area and Severity Index score; TIL: tildrakizumab. Adapted from Thaçi et al.11
These data show that tildrakizumab, at either 200 mg or 100 mg doses, not only demonstrated high levels of efficacy, but that efficacy could be maintained through 5 years in the majority who had achieved an initial response.
Long-term results are of particular relevance when prescribing a treatment for psoriasis because it is a chronic condition. Maintenance of a PASI score <3 indicates a clinically meaningful degree of disease control.12
Safety of Tildrakizumab Over 5 Years
Long-term safety data are crucial to inform clinical decision making in chronic diseases such as psoriasis. The adverse events of special interest were severe infections, confirmed extended MACE, and malignancies. Additionally, as there is a growing population of those aged ≥65 years, safety was specifically assessed in older trial participants.
In these analyses,13-16 safety data were available for all study participants who entered the 4-year extension period of reSURFACE 1 (≤256 weeks) or reSURFACE 2 (≤244 weeks). Throughout the time course of the trials, participants may have been switched from one tildrakizumab dose to another and therefore, some participants could be included in more than one group. Overall, 928 patients who had received 200 mg tildrakizumab and 872 who had received 100 mg tildrakizumab were included. Baseline characteristics were mean age 45.9 and 45.7 years, respectively (SD: 13.3 and 13.3); 71.0% and 71.8% male; and mean PASI score at baseline of 20.2 and 20.0 (SD: 7.9 and 7.7).
Exposure-adjusted incidence rates (EAIR) (i.e., events per 100 patient-years of exposure) were reported for all participants. Placebo and etanercept groups were not reported in these analyses.
Low Incidence of Severe Infections Over 5 Years
Doctor Andreas Pinter
Some, but not all, biologic treatments have been associated with higher risk of severe infections in people with psoriasis.16 With this in mind, it was of particular interest to assess the long-term incidence of severe infections in the reSURFACE 1 and 2 cohorts. Severe infections were defined as any infection meeting the regulatory definition of a serious adverse event (SAE), and/or requiring intravenous antibiotics, irrespective of whether it was reported as an SAE.13
Over 5 years of treatment, the EAIR for severe infections was 1.3 events per 100 patient-years of exposure (37 SAE) in the TIL 200 group and 1.2 in the TIL 100 group (34 SAE), with no meaningful difference between the two tildrakizumab doses. Diverticulitis was the most frequently reported SAE (EAIR: 0.25 in TIL 200; 0.22 in TIL 100), followed by pneumonia (EAIR: 0.18 and 0.15, respectively), cellulitis (EAIR: 0.15 and 0.15), and appendicitis (EAIR: 0.07 and 0.15). All other infections had a maximum EAIR of 0.11, with the majority having an EAIR of 0.04 (one SAE).
As biologics modulate the inflammatory cascade, they may impair the ability of the immune system to fight infections. However, it can be seen from these results that the EAIR for severe infections with tildrakizumab was low over this 5-year treatment period. Importantly, no new safety signals were reported at Weeks 244/256 compared with previous time points, showing that the risk remained low over long-term treatment.8,9
Low Incidence of Confirmed Extended Major Adverse Cardiovascular Events Over 5 Years
Professor Kristian Reich
In people with psoriasis, especially in moderate-to-severe cases, there is an increased risk of cardiovascular disease and related mortality.17,18 While a direct link between the two pathologies is neither known nor definite, it has been postulated that the systemic inflammation found in people with psoriasis may contribute to inflammatory damage linked with cardiovascular injury.19 This means that not only may comorbidities be more prevalent in those with psoriasis compared with the general population,20 but that this may necessitate a more frequent need for comedication.
In the present post hoc analyses, prespecified confirmed extended MACE included nonfatal myocardial infarction, nonfatal stroke, unstable angina, coronary revascularisation, resuscitated cardiac arrest, and cardiovascular deaths confirmed as ‘cardiovascular’ or ‘sudden’.14
Overall EAIR of confirmed extended MACE was 0.7 (20 events) for TIL 200 and 0.5 (14 events) for TIL 100 groups. The most reported confirmed extended MACE for TIL 200 and 100 groups, respectively, was acute myocardial infarction (EAIR: 0.15 and 0.07). Other confirmed extended MACE included coronary artery disease (EAIR: 0.15 and 0.04), angina pectoris (EAIR 0.07 and 0.04), cerebrovascular accident (EAIR: 0.04 and 0.07), and coronary artery stenosis (TIL 200 only: EAIR: 0.11). All other confirmed extended MACE had an EAIR of 0.04 (one occurrence). Only one death was recorded (EAIR: 0.04), in the TIL 100 group.14
The authors concluded that over 5 years of maintenance treatment with tildrakizumab, there was a low rate of EAIR of confirmed extended MACE in this cohort. Of note, over this extended time period, no dose-related impact on the incidence of MACE was observed. As there was an association between psoriasis and MACE,17 the long-term safety data are of particular importance to physicians and patients alike. A recent review of Phase III trials of IL-23p19 inhibitors found this class was not associated with increased rates of MACE and suggested that by controlling the inflammatory pathways that lead to psoriasis, such adverse events may potentially also be avoided, though this has yet to be confirmed.21
Low Incidence of Malignancies Over 5 Years of Treatment
Professor Jo Lambert
In people with psoriasis, malignancies, most notably lymphoma and nonmelanoma skin cancers (NMSC), may occur at a higher rate than those without psoriasis. However, direct links between incidence of cancer and both psoriasis itself and therapies have yet to be established.16,22 As such, this is another important safety aspect to monitor in any trial of a psoriasis-targeting therapy.
Over 5 years of treatment, EAIR of malignancies excluding NMSC were similar in the TIL 200 (0.6; 17 events) and TIL 100 (0.7; 20 events) groups. Reports of individual malignancy types (excluding NMSC) were low, with both malignant melanoma in situ and rectal adenocarcinoma having an EAIR of 0.07 for both TIL 200 and TIL 100 groups. An EAIR of 0.07 was described for pancreatic carcinoma in the TIL 200 group only and for bladder cancer in the TIL 100 group. All other cancer types (excluding NMSC) had an EAIR of 0.04 (one event).15
The EAIR of NMSC was also investigated. These were similar in the TIL 200 (EAIR: 0.4; 11 events) and TIL 100 (EAIR: 0.42; 12 events) groups. The most common NMSC for TIL 200 and TIL 100 groups was basal cell carcinoma (EAIR: 0.29 and 0.26, respectively). Other NMSC were squamous cell carcinoma of the skin (EAIR: 0.07 and 0.11); Bowen’s disease (EAIR: 0.04 and 0.11), keratoacanthoma (TIL 200: EAIR 0.04), and carcinoma in situ of the skin (TIL 100: EAIR 0.04). The ratio of squamous cell carcinoma to basal cell carcinoma was similar to that shown in the general population23 and with ustekinumab.24
The 5-year time point examined in this analysis was of particular importance as, while a drug-related infection may occur at any point during treatment, it would be expected that malignancies may be slower to manifest. A low rate of malignancies was shown over 5 years, with no dose-related occurrences. Previous analyses of these data found comparable rates to those who had received a placebo, adding to the evidence that tildrakizumab is not associated with increased malignancy development over time.
Long-term Safety of Tildrakizumab in Patients over 65 Years of Age with Moderate-to-severe Plaque Psoriasis Does Not Differ from the Overall Population
Professor Peter Van de Kerkhof
People ≥65 years of age with psoriasis are of particular interest and concern in studies of psoriasis therapies due to a higher proportion of comorbidities, especially MACE-associated, in this age group compared with those aged <65 years.25 With this in mind, the 256-week safety data of patients aged ≥65 years of age were analysed, which included 82 people in the TIL 200 (221.90 patient-years) and 79 in the TIL 100 (200.72 patient-years) groups. The mean age was 68.4 years in the TIL 200 group and 70.1 years in the TIL 100 (SD: 3.3 and 4.7, respectively). More participants in both groups were male (58.5% and 64.6%, respectively), with a mean PASI score of 20.3 and 19.4, respectively, at baseline (SD: 7.9 and 6.1).26
The overall EAIR for severe infections in the TIL 200 and TIL 100 groups were 2.70 (six events) and 2.99 (six events), respectively. Such infections almost exclusively had an EAIR of 0.45 for TIL 200 (appendicitis, hepatitis E, herpes zoster, peritonitis, pneumonia, and sepsis) and 0.50 for TIL 100 (bronchitis, cellulitis, infective arthritis, staphylococcal-related septic arthritis, and wound infection). Only gastroenteritis occurred in both TIL 200 (0.45) and TIL 100 (1.00) groups. While this was slightly higher than the overall study population,13 EAIR were still low, though, of note, these should be viewed in terms of the small sample size and limited number of patient-year exposures.
There were few cases of confirmed extended MACE in those aged ≥65 years, especially compared with the whole study population (n=1,800).14 EAIR were higher in those receiving TIL 200 (overall 1.35: 0.90 for coronary artery disease; 0.45 for coronary artery stenosis) than in those receiving TIL 100 (0.50 for respiratory arrest).
EAIR of malignancies (excluding NMSC) were higher compared to the general study population, but still relatively low.15 Overall EAIR were 2.70 (six events) for TIL 200, 1.99 (four events) for TIL 100; EAIR of NMSC were 2.70 (six events) and 2.99 (six events), respectively. Only bladder transitional cell carcinoma and rectal adenocarcinoma appeared in both TIL groups (EAIR of 0.45 and 0.50 for TIL 200 and 100, respectively). Malignancies appearing only in the TIL 200 group (EAIR 0.45) included colon cancer, endometrial cancer, metastatic breast cancer, and pancreatic cancer, with diffuse large B-cell lymphoma and endometrial adenocarcinoma occurring with an EAIR of 0.50 in the TIL 200 group.
Worldwide, people are generally living longer, leading to a higher percentage of those aged ≥65 and older. As such, investigation of adverse events in the older participants in the reSURFACE 1 and 2 trials is of particular relevance.22 A recent study of older patients receiving treatment for psoriasis including biologics found a higher rate of adverse events than reported for younger patients, though this study did not include any of the IL-23p19 inhibitors. The authors noted that this population also included many with comorbidities, especially those related to MACE.27 However, in the 5-year analysis of tildrakizumab presented here, safety results were similar to those reported in the entire cohort,13-15 with no dose-related increases in the rate of adverse events.
Conclusion
Tildrakizumab is a specific IL-23p19 inhibitor approved for the treatment of moderate-to-severe plaque psoriasis. In earlier analyses of the reSURFACE 1 and 2 trials, the efficacy of tildrakizumab in achieving and maintaining control of psoriasis over 244 weeks was high and significantly more than with placebo or etanercept,8,9 establishing tildrakizumab as a suitable treatment option for the long-term control of psoriasis.
The 5-year data presented here show that with tildrakizumab administered every 12 weeks, maintenance of control (PASI score <3, and in many cases <1), is not only achievable, but maintained over the long term. This is important as psoriasis is a condition that is associated not only with the pain and irritation, but also with myriad, life-limiting social and psychological effects.28,29 As such, people with psoriasis want a therapy that will safely provide long-term disease control.2,3
A major concern for those receiving a psoriasis therapy is whether it is safe when chronically administered.2,3 Of note, IL-23p19 inhibitors have not been shown to increase safety concerns when used for long-term control of psoriasis.20 In the present analyses, there were low rates of severe infections, extended MACE, and malignancies over 5 years of treatment with both tildrakizumab doses, with no new safety signals. The findings of these new analyses are aligned with previously published 148-week results that have also described long-term disease control and reported low rates of EAIR of adverse events, lower than in the placebo or etanercept 50 mg groups, and that few participants discontinued tildrakizumab because of a drug-related SAE.8 Taken together, these data should be taken into consideration when choosing a long-term therapy for psoriasis.
For people ≥65 years of age, there were low rates of SAE and no dose-related increases of SAE; importantly, these were comparable with the overall population.13-15 This result is of particular importance because of the greater rates of comorbid conditions found in this age group22,24 and, as such, may add tildrakizumab to the therapies considered for older patients with psoriasis.
In summary, the data presented here confirm the long-term efficacy and safety of tildrakizumab for treatment of moderate-to-severe psoriasis.