Meeting Summary
The symposium “IL-23 Inhibition: From Pathophysiological Jungle to Clinical Clearance” took place during the 2019 annual European Academy of Dermatology and Venereology (EADV) congress in Madrid, Spain. The presentations gave an overview of how to navigate the complexities of the psoriasis treatment landscape, including updates on the newest developments in psoriasis, from pathophysiological considerations to clinical relevance, with a focus on how insights from recent trials can be applied in daily clinical practice.
Prof Reich discussed the pathophysiology of psoriasis and the scientific rationale for using different classes of biologics. It is likely that IL-17 and IL-23 have differential roles in psoriasis and psoriatic arthritis (PsA) disease domains, and these different mechanistic roles translate into differences in clinical behaviour of respective inhibitors.
Analyses of clinical trial data, as presented by Prof Warren, show that treatment with IL-23 inhibitors results in high levels of efficacy that can be maintained for up to 3 years, with extended maintenance of 90% reduction in the Psoriasis Area and Severity Index (PASI) 90 responses after treatment withdrawal. Furthermore, the majority of patients report improvements in quality of life during treatment, with improved Dermatology Life Quality Index (DLQI) scores after 1 year of treatment. IL-23 inhibitors are a safe treatment option for patients with psoriasis, as evidenced by data produced by long-term extension and randomised clinical trials.
Prof Kirby shared his experiences managing patients with specific clinical challenges and comorbidities, such as PsA, obesity, cardiovascular diseases, psychological disorders, and inflammatory bowel disease (IBD). Current evidence indicates that IL-23 may be an attractive treatment target for disease and comorbidity management. A multidisciplinary approach to the management of psoriasis and its associated comorbidities is therefore recommended.
A Guide to Navigate Through the Jungle: Finding the Best Targets for Psoriasis Professor Kristian Reich
Insights into psoriasis pathophysiology have led to the development and expansion of cytokine-targeted therapies. In the early and mid-2000s, the search for effective treatments led to the development of the first biologic treatments for psoriasis, including the anti-TNF therapies etanercept, infliximab, and adalimumab. More recent developments of the anti-IL treatments acting on the IL-23/12 (ustekinumab), IL-17 (secukinumab, ixekizumab), and IL-23 (guselkumab, tildrakizumab, risankizumab) pathways have shown that a more targeted approach may be the answer to more optimised psoriasis treatment; however, no single treatment is ideal for all patients with psoriasis.
The complicated evolution of the psoriasis disease model shows that feed-forward and feed-backward responses are both involved in the inflammatory process behind keratinocyte proliferation, driven mainly by T-cell activation (Figure 1).1
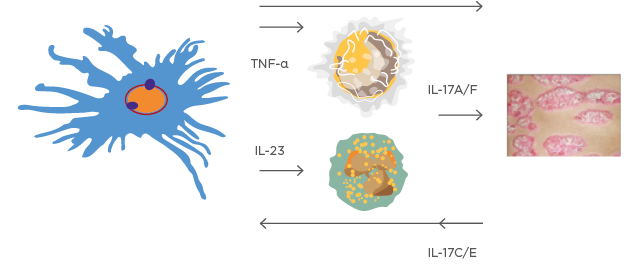
Figure 1: A very simplified version of the ‘cytokine soup’ model.
This model illustrates how dendritic cells, T-cell activation, and several feed-forward and feed-backward processes are involved in psoriasis development.
Images courtesy of Prof Kristian Reich.
Closer examination of the IL-17 pathway reveals that several IL-17 ligand and receptor family members, including IL-17A, IL-17F, and IL-17C, are largely involved in the development of psoriatic lesions.2 Furthermore, IL-17A is a main activator of abnormal epidermal function in TNF-primed keratinocytes.3 This has led to the development of the IL-17 blockers secukinumab, ixekizumab, and bimekizumab, and the IL-17A receptor blocker brodalumab,4 which show similar efficacy to other currently available treatments.5-13
The inflammatory nature of psoriasis means that patients may develop other comorbid diseases such as PsA. A mechanistic study of skin and joints showed that IL-17 and TNF-α activation not only increased the production of T cells and keratinocytes, but also increased the production of osteoprotegerin, a soluble decoy receptor for receptor activator of NFκB ligand, and osteoclast progenitor cells, which play a role in bone resorption.14 An examination of the genetics underlying psoriasis development revealed the involvement of multiple loci, leading to abnormal cytokine responses, including IFN, NFκB, as well as IL-17 and IL-23 receptor signalling.15
Current models theorise that IL-23 can significantly activate pathogenic IL-17 production, but that IL-17 produced independently of IL-23 is physiologically normal. Therefore, IL-17 blockade may result in oversuppression of the IL-17 pathway in patients with psoriasis.16,17 The differential effects of IL-17 and IL-23 show that blockade of IL-23 ameliorates colitis symptoms and improves epithelial barrier integrity in patients with IBD, while IL-17 blockade exacerbates disease symptoms, causing epithelial barrier breakdown and leaking of the lumen contents.18
Furthermore, patients with psoriasis have been shown to be at higher risk of developing IBD compared with healthy controls.19 Psoriasis is also associated with the development of several comorbidities, including PsA, anxiety, and depression. Treatment with guselkumab, a human monoclonal antibody against the p19 subunit of IL-23, yielded greater improvements in anxiety and depression in patients with moderate-to-severe plaque psoriasis, compared with placebo or adalimumab treatment.20
One of the most pressing current challenges in the treatment of psoriasis is the achievement of disease remission, which is often hampered by ‘disease memory’, characterised by the presence of T cells with tissue-resident memory T cell (TRM) phenotypes in clinically non-active psoriatic lesions.21 These TRM cells are capable of maintaining IL17 production and may be the main drivers behind disease recurrence.21 However, data from the VOYAGE 2 study with guselkumab show that 86.0% of patients receiving guselkumab maintained a PASI 90 response from Week 28 to Week 72, compared with 11.5% of patients in the withdrawal group.22 Furthermore, maintenance of a complete (PASI 100) response after drug withdrawal was associated with the continued suppression of IL-17A, IL-17F, and IL-22, reducing the levels of these cytokines to levels similar to controls.22 These long-term responses may be linked to several markers, including shorter disease duration, lower baseline IL-17F levels, PASI 100, and Investigator’s Global Assessment Score 0 responses at Week 28 of treatment, and higher guselkumab levels at Week 28 of treatment.23
In conclusion, IL-17A and IL-17F are the key activators of abnormal epidermal function in TNF-primed keratinocytes. IL-23 is the master cytokine, activating pathogenic Th17 activity and having possible effects on other cells in the pathway. It is likely that IL-17 and IL-23 have differential roles in psoriasis and PsA disease domains. These different mechanistic roles translate into differences in clinical behaviour of respective inhibitors.
Mapping Out the Evidence: What Do the Data Say?
Professor Richard Warren
Prior to the 1980s, it was believed that psoriasis was driven by dysregulation of keratinocyte hyperproliferation, after which, the role of T cells and the Th1/Th2 paradigm evolved.
The discovery of the IL-17 pathway led to insights into the involvement of the IL-12, IL-23, and IL-17 cytokines.24 This opened the door for the development of inhibitors acting on these pathways, with increasing focus on IL-17A and the p40 and p19 subunits of IL-23.24
Several IL-23 inhibitors are currently available in the USA and the European Union (EU), including guselkumab, tildrakizumab, and risankizumab, with possible approval of mirikizumab in the next few years (Figure 2). Clinical trial data from the reSURFACE 1 and reSURFACE 2 studies show that IL-23 inhibition with tildrakizumab led to the maintenance of a 75% reduction in PASI (PASI 75) response in approximately 65% of patients at Week 12, and a PASI 90 response in almost 60% of patients at Week 28, compared with placebo and etanercept.25 A similar proportion of tildrakizumab responders retained this response through Week 148 of treatment.26
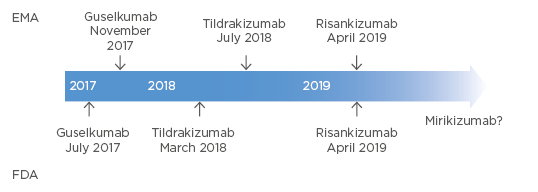
Figure 2: Approvals of IL-23 inhibitors in the EU (EMA) and the USA (FDA).
EMA: European Medicines Agency; EU: European Union; FDA: U.S. Food and Drug Administration.
Treatment efficacy and maintenance of response have been demonstrated in several studies. The results from the VOYAGE 1 and VOYAGE 2 clinical trials show that PASI 90 response was achieved in >70% of patients treated with guselkumab at Week 16, and maintained in 80% of patients at Week 48 and through Week 156.27,28 These responses were also maintained in almost 50% of patients up to 6 months after withdrawal of guselkumab; 11.5% of patients still maintained a response 52 weeks after withdrawal.22 The majority of patients regained a PASI 90 response following retreatment with guselkumab.22 Data from the UltIMMa-1 and UltIMMa-2 trials further underline the role of the IL-23 pathway in psoriasis management; 75% of patients treated with risankizumab achieved a PASI 90 response at Week 16, and approximately 80% of patients achieved PASI 90 responses within the first year of treatment.29 Furthermore, data from the IMMvent study showed that IL-23 inhibition with risankizumab led to a PASI 90 response in >70% of patients at Week 16 and Week 44.30 Data from the IMMhance study showed that these responses were maintained in over 50% and in over 4% of static Physician’s Global Assessment 0/1 responders through Weeks 52 and 104, respectively, after withdrawal from risankizumab at Week 16.31
Importantly, patient-reported outcomes on quality of life during treatment appear to mirror the clinical trial outcomes; patients receiving tildrakizumab reported improvements on the DLQI from baseline to Week 52, which correlated with improved PASI scores.32 Furthermore, approximately 75% of patients treated with guselkumab have reported DLQI 0/1 scores that were improved and maintained from Week 76 to Week 156 of treatment.28 Data from the UltIMMA-1 and UltIMMA-2 trials show that patients receiving risankizumab reported improved DLQI 0/1 scores at Weeks 16 and 52.33
The results of several studies also demonstrate the safety of IL-23 inhibitors, with no new or unexpected safety signals for tildrakizumab, no safety signals evident with continued use of guselkumab, and no new safety signals for risankizumab.26,29,34
But how does the efficacy of IL-23 inhibitors compare with that of IL-17 inhibitors in psoriasis management? In the ECLIPSE trial, the first head-to-head comparison of an IL-23 inhibitor (guselkumab) and an IL-17 inhibitor (secukinumab) showed that 84% of patients receiving guselkumab achieved the primary endpoint of a PASI 90 response at Week 48 of treatment compared with 70% of patients in the secukinumab group.35 Both drugs showed a safety profile similar to their registrational trials.35 However, the real test will be to see how long-term treatment with IL-23 inhibitors performs in real-world situations, though early data are promising.
In conclusion, treatment with IL-23 inhibitors results in high levels of efficacy that can be maintained for up to 3 years, with extended maintenance of PASI 90 responses after treatment withdrawal. Furthermore, the majority of patients report improvements in quality of life during treatment, with DLQI scores of 0/1 after 1 year of treatment. IL-23 inhibitors are a safe treatment option for patients with psoriasis, based on randomised clinical trial data and long-term extension studies. In a head-to-head comparison study, guselkumab showed superior efficacy, compared with secukinumab, in the primary endpoint at Week 48.
Tips and Tricks for the Expedition: Beyond the Skin Surface
Professor Brian Kirby
Successful psoriasis management does not solely depend on the treatment of the skin manifestations of psoriasis, as the presence of several comorbidities, including PsA, obesity, IBD, cardiovascular complications, psychological disorders, and psoriasis in difficult-to-treat or high-impact areas all represent further treatment challenges for clinicians. However, data from several studies currently show that treatment with the IL-23 inhibitor guselkumab is effective in improving psoriasis of the hands, feet, and scalp, and palmoplantar pustulosis, compared with adalimumab and placebo, respectively.36,37
The role of psoriasis in the development of PsA has been examined in several studies, showing that psoriasis occurs in 6–48% of psoriasis patients,38 with a probable prevalence of up to 30% in psoriasis patients and high percentages of underdiagnosis (Figure 3).39,40 However, early diagnosis and treatment with disease-modifying drugs has a substantial impact on long-term morbidity.41-43
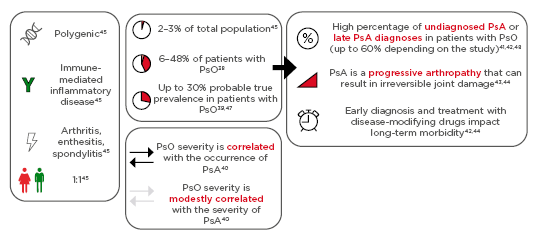
Figure 3: Psoriasis and psoriatic arthritis.
PsA is a polygenic, immune-mediated inflammatory disease, frequently occurring with arthritis, enthesitis, or spondylitis, with equal occurrence rates in males and females.38-48
PsA: psoriatic arthritis; PsO: psoriasis.
Therefore, dermatologists play a key role in the early diagnosis of psoriasis, and in the prevention of dactylitis, enthesitis, and joint destruction.49 Current guidelines recommend consultations with other specialists when both psoriasis and PsA coexist, emphasising the importance of a multidisciplinary approach to disease management.50,51 The use of questionnaires such as the Psoriasis Epidemiology Screening Tool (PEST) can help clinicians with diagnosis.52,53 Regarding treatment, clinical trial results show that 58% of patients treated with the IL-23 inhibitor guselkumab achieved American College of Rheumatology (ACR) 20 scores at Week 24 of treatment compared with 18% of patients receiving placebo. Similarly, 79% of patients receiving guselkumab achieved PASI 75 scores at Week 24 of treatment compared with 13% of patients receiving placebo.12
The link between psoriasis and obesity can be explained by a dual-compartmental model of inflammation, as patients with psoriasis are at risk of increased BMI, and increased prevalence of obesity and metabolic syndrome. Conversely, patients with a high BMI or obesity are at high risk of psoriasis, with a risk of decreased efficacy of biologic treatment.54-56 The results of the ECLIPSE study, in which patient weight was balanced between treatment groups, showed that guselkumab treatment resulted in a PASI 90 response in >80% of patients, across weight quartiles and BMI at Week 48.57 Furthermore, the results of the VOYAGE 1 and VOYAGE 2 studies showed consistent maintenance of response across demographic subgroups in patients with psoriasis who were treated with guselkumab for up to 3 years.58
Psoriasis correlates with alcohol abuse, depression, anxiety, and cardiovascular disease, which can often be successfully managed by simultaneously treating both the skin condition and the psychological symptoms.59,60 Patients treated with guselkumab have shown reductions in anxiety and depression over time, as measured by the Hospital Anxiety and Depression Scale-anxiety (HADS-A) and HADS-depression (HADS-D) scales.20 Therefore, patients with psoriasis should always be screened for psychological comorbidities, including excessive alcohol use.
The significant association between psoriasis and IBD may be due to genetic abnormalities, immune dysfunction, systemic inflammation, or dysregulation of gut microbiota.24,61,62 Targeting IBD with IL-23 inhibitors has shown promise in clinical trials; the results of the IM-UNITI trial show that induction and maintenance treatment with ustekinumab resulted in significant levels of clinical remission, clinical response, and glucocorticoid-free remission in patients with Crohn’s disease at Week 44 of treatment.63 Risankizumab treatment has also resulted in increased levels of CR100 and clinical remission in patients with Crohn’s disease.64
In conclusion, patients with psoriasis are at higher risk of developing comorbidities such as PsA, obesity, cardiovascular diseases, psychological disorders, and IBD (Crohn’s disease and ulcerative colitis). Current evidence indicates that IL-23 may be an attractive treatment target for disease and comorbidity management, and treatment of the skin condition often leads to improvements in associated comorbidities. A multidisciplinary approach in the management of psoriasis and its associated comorbidities is recommended.
WATCH THE FULL SYMPOSIUM ONLINE: https://youtu.be/g0COM5QTDAo
Date of preparation: November 2019
CP-125297







