Meeting Summary
Several sessions examined the landscape of mycosis fungoides and the efficacy of chlormethine gel (CL gel) in all stages of mycosis fungoides cutaneous T-cell lymphoma (MF-CTCL), looking at the tolerability and ease of use of CL gel alongside other comorbidity medications and treatments. Evidence from real-world use in early-stage disease shows that CL gel, also known as mechlorethamine or nitrogen mustard, is well-tolerated and the non-systemic absorption leads to the hypothesis that drug interactions are unlikely to happen. Real-world evidence also revealed how most of the practitioners examine the body surface area (BSA) as an outcome measure of treatment response rather than the modified Severity-Weighted Assessment Tool (mSWAT), Physician’s Global Assessment (PGA), or the Composite Assessment of Index Lesion Severity (CAILs). Improved health-related quality of life (HRQoL) was observed in responders compared with nonresponders across several investigations. In comparison, the use of topical steroids presented at this meeting as monotherapy is also covered in this review. ‘By-time analysis’ is a new statistical approach used to perform a post-hoc analysis of the 201 study and to analyse the PROVe study data.
INTRODUCTION
MF-CTCL is a rare type of cutaneous non-Hodgkin’s lymphoma where uncontrolled growth of the pathological skin-homing T-lymphocytes results first in skin alteration.1 Since this disease is usually indolent, most patients live a normal lifespan. The global incidence of primary cutaneous lymphoma rose from 5 per million in 1980–1982 to 14.3 per million in 2001–2003. In 2004–2005, the incidence was 12.7 per million.2 Although it is the most common subtype of CTCL, MF-CTCL is still a rare disease. In the USA, the annual incidence rate is 4.5 cases per million or 0.55 per 10,000 person-years (age-adjusted to the US Year 2000 Population Standard).2 The incidence of MF-CTCL in Europe is currently unknown (prevalence per million in European Union [EU] = 80), although Orphanet provides an estimated annual incidence of MF and variants of between 1 per 350,000 and 1 per 110,000.3 MF-CTCL accounts for approximately 70% of CTCL and in the UK, there are approximately 450 new diagnoses each year.3 An evaluation of 1,502 MF patients using the International Society for Cutaneous Lymphomas (ISCL)/EORTC revised staging proposal revealed that median survival in early-stage IA and IB patients was 35.5 years and 21.5 years, respectively, versus 1–5 years for later stages.4
Key studies were discussed across several sessions, the results of which indicated how CL gel can be placed in the therapeutic strategy of treating MF-CTCL.
CHLORMETHINE GEL: NOT ONLY A NEW FORMULATION
On the first day of the congress, the satellite symposium ‘Tried and Applied: experiences with CL gel in MF-CTCL’, held by Helsinn, provided an introduction to CL gel and an overview of treatment management of MF-CTCL.
Overview of Chlormethine Gel
Professor Evangelia Papadavid
Prof Papadavid opened the session explaining how a weapon of war had become an effective anticancer treatment, and since the 1950s through to the present day, topical chlormethine (also known as mechlorethamine, nitrogen mustard) has been used efficiently as a skin-directed therapy (SDT) to treat MF-CTCL.5,6
Prof Papadavid presented a novel formulation of CL gel (Valchlor® in the USA;7 Ledaga® in Europe).8 It was developed in 2004 and, on the basis of the largest clinical trial ever conducted in MF-CTCL (Study 201, Lessin et al.),9 CL gel was approved in the USA in 2013 (Valchlor),7 in Israel in 2016 (Ledaga), and in Europe in 2017 (Ledaga). CL gel has differing indications in the USA versus Europe, with Europe suggesting CL gel is appropriate for use in adult patients of MF-CTCL, while in the USA and Israel the indication is specific to Stage IA and IB as this was the major patient population evaluated in the pivotal trial. Recordati Rare Diseases is registering and launching the gel formulation in many countries at world-wide level (except the USA, Israel, and China). A topical treatment needs to be practical for ease of use and, characteristically, CL gel formulation meets this goal.
It contains two major components: Transcutol®10-12 and Klucel®.13 Transcutol (diethylene glycol monoethyl ether [a galenic formulation to optimise absorption]) can promote drug delivery to the epidermis creating an intracutaneous depot. When applied to the skin it is non greasy, quick-drying, colourless, self-administrable, and easy to use, but flammable. In the EU, the gel can be stored for 60 days at 2–8 °C,8 while up to 90 days according to the USA label.7
Considering the role of CL gel in the treatment landscape of MF-CTCL, Prof Papadavid continued with an overview of chlormethine within the clinical guidelines. In Europe,14,15 all SDT are recommended equal ranking, but topical chlormethine is recommended as a first-line SDT starting from early-stage MF-CTCL and including maintenance therapy.14 Similarly, the British Association of Dermatologists (BAD) and the UK Cutaneous Lymphoma Group (CLG) state that mechlorethamine/chlormethine as SDT is recommended for use in the treatment of all stages of the disease16 and in maintenance. CL gel is therefore currently endorsed by many international guidelines for use in MF-CTCL adult patients.
Chlormethine Pivotal Results: Study 201 (Lessin et al.)9
Professor Julia Scarisbrick
Prof Scarisbrick presented an overview of what has been learned from the pivotal Phase II/III, multicentre, randomised, observer-blinded, noninferiority trial known as Study 201.9 The study assessed the efficacy and safety of this novel chlormethine as a gel (0.02%) in the treatment of MF-CTCL in adults over a period of 12 months, after which an extension study (Study 202)17 followed for 7 months to evaluate patients who did not completely respond to treatment during Study 201, and who continue to be treated by CL gel at 0.04% w/w.
Efficacy Data
CAILs (primary endpoint), mSWAT, and BSA (secondary endpoint) analyses reported that CL gel was noninferior to chlormethine ointment (N=260). Response rates for the CL gel were consistently higher than those seen with the CL ointment for the primary endpoint of CAILs, for both intent-to-treat (ITT) and efficacy-evaluable (EE) populations(in patients having received the treatment for at least 6 months. This difference was statistically significant for the EE population (CL gel 76.9% versus CL ointment 58.9%; p=0.011) (in patients having received the treatment for at least 6 months. Among the secondary endpoints, analysis of time-to-response demonstrated superiority of CL gel to CL ointment.9 A significantly faster time to response was shown for CL gel than CL ointment; 50% of patients demonstrated a CAILs response at 26 weeks for CL gel versus 42 weeks for CL ointment. There was a similar duration of response for both interventions. In Study 202, while considering the effects of dosage, findings revealed a longer duration of treatment with CL gel may result in an improved response rate.17 The result indicates that this is an effective treatment for patients with MF-CTCL (Figure 1).
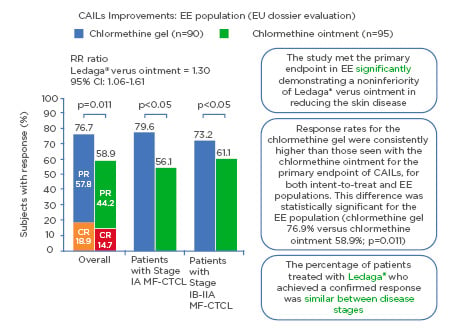
Figure 1: Composite Assessment of Index Lesion Severity improvement: efficacy evaluable population (European Union dossier evaluation).
Response rates highlighting how the primary endpoint of noninferiority of Ledaga® (Helsinn Birex Pharmaceuticals [HBP] Ltd., Dublin, Ireland) versus chlormethine ointment was met with ≥50% improvement in skin lesion severity with Ledaga.8
CAILs: Composite Assessment of Index Lesion Severity; CI: confidence interval; CR: complete response; EE: efficacy evaluable; EU: European Union; MF-CTCL: mycosis fungoides cutaneous T-cell lymphoma; PR: partial response; RR: response rate.
Adapted from Lessin et al.9
Safety data
The main adverse event (AE) was skin irritation including pruritus, dermatitis, and skin infections, with a significantly higher incidence found in the gel arm of the study (p=0.040); the difference was due primarily to an increased incidence in the gel arm of moderate-moderately severe (Grade 2 or 3) local dermal irritation. In most cases, this local tolerability can be effectively managed with temporary suspension of treatment/reduced frequency such that patients can return to treatment to maximise response potential. However, these events were managed with the protocol-directed treatment adjustments. Similar proportions of patients withdrew from the study due to skin irritations (CL gel 20.3% versus CL ointment 17.3%). Prof Scarisbrick pointed out that the use of topical dermocorticoids was not allowed. No severe AE related to the gel were reported, nor were there any detectable levels of chlormethine observed in the blood. Development of three nonmelanoma skin cancers (versus eight in the ointment arm) was considered unrelated to the use of CL gel. Approximately 60% of patients experienced at least one AE and these numbers were comparable for both gel and ointment arms. No AE were deemed to be serious and a total of 20% of patients dropped out of the study due to AE experienced.
Previous reports showed that patients who developed a skin reaction went on to report good responses with continued treatment,6,18,19 which may be an indication that skin rashes experienced with CL gel could be associated with a better outcome.
Although approximately half of all patients started on CL gel will develop an irritant skin reaction, the aetiology of that skin reaction is unknown. This symptom is not unique, a similar result was observed in the PROVe study20 (Prospective, Observational, US-based study) which assessed efficacy outcomes, AE, treatment patterns, and QoL in patients diagnosed with MF-CTCL and treated with CL gel based treatment (Valchlor).
It is known that topical corticosteroids improve the cutaneous reactions, but there may also be a question of whether they subsequently reduce the efficacy of the treatment. For chemotherapeutic agents such as fluorouracil (5FU)21 and imiquimod,22 the inflammatory response contributes to the destruction of the tumour cells. Therefore, an important question must be, if by dampening down the inflammatory response, do the topical corticosteroids also reduce the efficacy of the anticancer treatment?
An Exploration of CL Gel-Induced Skin Reactions
Prof Scarisbrick also presented details of an EORTC trial dedicated to study the CL gel-related skin reactions, the REACH study (Rash Etiology After CHlormethine gel). It will address pertinent research questions: the cause of true skin reactions, whether the response rate is improved in patients who developed a skin drug reaction, and whether patients should continue treatment on a reduced dosing schedule. The addition of topical corticosteroids in those patients unable to tolerate the treatment even at reduced dose will also be investigated for safety and efficacy. Eligibility criteria for early-stage patients include no previous exposure to CL gel or other chlormethine compound, and the primary endpoint will be the overall response rate across three groups of patients defined according to treatment dose and response.
New Analysis of Study 201 Efficacy: The ‘By-Time Analysis’
Professor Larisa Geskin
Prof Geskin presented a novel assessment of Study 201 performed with a new statistical approach named by-time analysis. The principle is to take a dynamic ‘picture’ of the responding patients at a specific time. This approach reflects the actual number of responders and nonresponders at a particular time point and may be reflective of the biological differences between the patients, underscoring heterogeneity of the disease even across the same stage. Classical evaluation of the response rate of a therapeutic strategy is a measure of the response independent of when the response occurred, and although this is also true of the by-time analysis, the latter is a reflection of each individual patient’s response to therapy at all evaluable time points.
Prof Geskin continued with a discussion of Study 201, reviewing that in a classic standard process of evaluation of responses, a responder needed to be confirmed over two consecutive visits to acquire the ‘confirmed responder status’; once the response was confirmed, the responder was always counted as a ‘responder’, even when the response was eventually lost. In the original 201 study, the assessment of response was conducted monthly between 1 and 6 months and bi-monthly between 7 and 12 months, over the course of 1 year. The current by-time post-hoc analysis included patients who had data available at each assessment timepoint. This new evaluation accounted exclusively for responses when they actually occurred and did not count a patient as a responder when the responses were lost. This precise focussing on responders at the time of response reveals that there are three groups within this ‘homogenous’ cohort of patients who may have inherent biological differences in their ability to tolerate CL gel and to respond to therapy.
The new by-time analysis revealed that there were patients who could be classified as early responders, late responders, and intermittent responders. Of note, there is a peak of responses in CAILs starting at 8 months which suggests that long-term therapy may be needed to adequately assess the optimal response to the treatment. Overall, this new analysis revealed similar overall response rates compared with the original analysis, but in addition it provided a new insight into the various timings of responses and provided a window into biological differences of the previously thought homogenous patient population in their disease course. A full manuscript is currently under preparation and data should be available by 2020.
PROVe STUDY
Professor Ellen J. Kim
Prof Kim presented preliminary results of the PROVe study.20,23 It is the largest real-world prospective observational study conducted in the USA, which began shortly after CL gel was approved by the U.S. Food and Drug Administration (FDA); it aims to examine how CL gel was used in daily clinical practice. A total of 298 patients were enrolled across 41 university and clinic-based centres with a 2-year follow-up, regardless of whether the gel was discontinued. The gel was used predominantly in early-stage disease according to the USA indication of CL gel, mainly in combination with other SDT or with systemic treatment, and most patients applied it once per day although there were a variety of dosing frequencies. It is important to emphasise that PROVe is an observational study with data acquired from real-world experience.
Baseline demographics of the patient population showed a median age of 62 years at the time of PROVe enrollment and the cohort was predominantly male (n=179, 60%; 68% Caucasian; 15% African American), and the duration of diagnosis prior to enrollment was 3 years. Approximately 68% were early-stage patients, although nearly 19% had no stage information included on the standard of care clinical visit information. A total of 93% had prior therapy including SDT, topical steroids, and systemic therapies. After 12 months, approximately 80% of patients were still on therapy and 63% had dose-frequency change during the study to help manage side effects. By the end of the study, 40% of patients had discontinued treatment.23
The PROVe study highlighted the response rates achieved in the IA–IB cohort by %BSA improvement at 1 year as well as preliminary Skindex-2924 data concerning HRQoL. Efficacy was also evaluated with a novel by-time analysis approach and examined the variation of BSA data over 24 months. A trend for improved responses was observed over the time. The median time on treatment was 32 weeks.
A key aim of the study was to measure QoL; for this, the Skindex-29 questionnaire24 was used to assess the three domains of symptoms, emotions, and functioning. Domain subscores were collated in an overall 100-point scale in which higher scores were associated with lower HRQoL or higher impact of disease. Over the 12/24-month period, weighted mean Skindex-29 scores for emotions, symptoms, and functioning were 26.4/26.4, 26.8/25.6, and 13.2/14.0 in responders; and 37.1/35.7, 34.8/35.6, and 22.8/22.6 in nonresponders, respectively. Differences in the QoL subscores between responders and nonresponders were all significant (p<0.001), favouring responders. This suggests that CL gel-based treatment as part of a treatment regimen is associated with improved HRQoL in patients who are experiencing a clinical response.
There were a variety of treatment regimens in the PROVe study that reflect general practice in the real world. For practitioners who regularly saw MF-CTCL patients, it was common to combine topical or systemic therapies reflected in the study. Real-world data also showed that although CL gel is approved for early-stage patients with MF-CTCL in the USA, it is also being used off-label in some advanced-stage patients. For concomitant therapy, the majority of patients were on SDT as well as CL gel. The most common concomitant therapy was corticosteroids, while many patients also received systemic therapies.23
Similarly to Study 201 there were no serious AE. According to public data available, 13% of patients (n=38) experienced dermatitis of all grades, with approximately 7% (n=22) reporting skin irritation with CL gel.23 A full manuscript summarising all the efficacy (using by-time approach) and safety data will be available in the near future.
Proclipi Database
Professor Julia Scarisbrick and Doctor Pietro Quaglino
Prof Scarisbrick, on different sessions of the congress, provided an update of the Prospective Cutaneous Lymphoma International Prognostic Index Study (PROCLIPI),25 a database of approximately 1,600 patients and 71 registered centres across 6 continents, the aim of which is to develop a prognostic index for MF-CTCL.26 A key success of PROCLIPI was in recruitment, which has exceeded the original planned recruitment of 1,000 early-stage and 500 advanced-stage patients over 5 years, with data collected over 10 years.
Most early-stage patients in the database are equally split between Stage IA and IB and approximately 8% of patients are Stage IIA. The median age of early-stage patients is younger than the patients with advanced disease (45–69 years versus 55–73 years) and patients with early-stage IA are younger and present earlier than those with Stage IB. The database holds information on the number of patients who have progressed to advanced-stage disease and the number of associated deaths. An investigation of the data held determined which patients were more likely to progress to advanced disease. A better prognosis was observed in early-stage patients who had no nodal involvement, as determined through imaging and biopsy.
Dr Quaglino also presented treatment data from the PROCLIPI database, identifying treatments performed in real life in early-stage MF-CTCL, and how response rates differ according to treatment and tumour-node-metastasis-blood stage. It appears there are inconsistencies with current guidelines, particularly in the use of systemic therapies, and it was found that a heterogeneous approach to treatment exists in practice.
Topical Corticosteroid Monotherapy
Doctor Saritha Kartan
Topical corticosteroids therapy widely used in MF-CTCL was studied as monotherapy by Dr Kartan (Philadelphia, Pennsylvania, USA). A paucity of objective data on the effectiveness of topical steroids as monotherapy exists. She presented a retrospective study from 2013–2019 which sought to quantify treatment response rates. Previous studies had shown that topical steroids serve as an effective treatment, but these studies did not define either the response rates or the characteristics of responders versus nonresponders, nor did they include higher stages of disease.27,28
Using changes in BSA and mSWAT with subgroup analysis on responders versus nonresponders for age, gender, and stage distributions, findings of this retrospective study revealed that 24% (39/163) of patients were on topical steroid monotherapy (mainly clobetasol) as an initial treatment. Of those 39 patients, 74% improved with an average 64% decrease of lesion in BSA and the remaining 26% either flared or did not improve with an average increase of lesion in BSA of 51%. Among responders, 54% (15/39) had a partial response (≥50% decrease in BSA), and 41% (12/29) had a complete response (BSA lesion: 0%). Stages IA and IB were highly representative of the responder group (among which 59% were IA; 34% were IB) who improved on steroids versus 60% not improved/flared (these were IA only); additionally, there were a variety of stages in the not improved/flared group including higher stages which did not improve. Most patients were over 60 years of age in both groups (improved group: 76%; not improved/flared group: 90%) and male gender was more prevalent in the not improved/flared group (not improved/flared group: 80%, versus improved group: 48%).
In summary, topical steroids alone can achieve complete remission in a limited subset of patients (females more so than males), and in very early-stage disease. Further studies considered will include a prospective, multicentre trial investigating the duration of response with topical steroids in monotherapy to see how long patients can sustain either a partial or complete response with continual use of topical steroids. A current investigation is also underway to identify clinical or histopathologic features in responders through evaluation of malignant clones, and to determine BSA lesion improvement with topical steroid monotherapy versus combination therapy with another SDT such as chlormethine.
Summary
By-time analysis results from both PROVe and Study 201 reveal the importance of a close follow-up of the patient and of continued treatment to enable them to reach the maximum treatment response. CL gel is a promising new medication in MF-CTCL, and skin reactions will be investigated to determine whether such reactions are indicative of a potential response as well as to help the management of this side effect. Finally, HRQoL studies reveal that the QoL data (including preliminary data from the PROVe study and presented within the EORTC-CLTF meeting) showed contribution to an improvement in responder patients. This important information for MF-CTCL patients encourages the administration of efficient treatment to those patients.







